Balance each of the following equations, and classify them as precipitation, acidbase, gas-forming, or oxidationreduction reactions. Show
Question:
Balance each of the following equations, and classify them as precipitation, acid–base, gas-forming, or oxidation–reduction reactions. Show states for reactants and products (s, ℓ, g, aq).
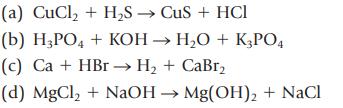
Transcribed Image Text:
(a) CuCl₂ + H₂S → CuS + HCl (b) H3PO4 + KOH → H₂O + K3PO4 (c) Ca + HBr →→ H₂ + CaBr₂ (d) MgCl₂ + NaOH → Mg(OH)₂ + NaCl
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a CuCl2 H2S CuS HCl This is a precipitation reaction because it forms a solid Cu...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Balance each of the following oxidation reduction reactions by using the oxidation states method. a. C2H6(g) + O2(g) CO2(g) + H2O(g) b. Mg(s) + HCl(aq) Mg2+(aq) + Cl2(aq) + H2(g) c. Cu(s) + Ag+(aq)...
-
A current computer-related incident or change in cybercrime law and write a details addressing the following: The potential cause for the incident/change in law How this incident/change in law...
-
Each of the following nucleophilic substitution reactions has been reported in the chemical literature. Many of them involve reactants that are somewhat more complex than those we have dealt with to...
-
The current zero-coupon yield curve of semi-annually compounded rates for risk-free bonds is as follows: 1.0 Years 1.5 Years 2.0 Years 2.5 Years 3.0 Years 9.00% 10.20% 3.00% 4.00% 6.20% MATURITY 0.5...
-
In our discussion of short-run exchange rate overshooting, we assumed that real output was given. Assume instead that an increase in the money supply raises real output in the short run (an...
-
Explain why the margin of error determines the accuracy with which a sample mean estimates a population mean.
-
Following data relate to an oil engine working on Otto cycle : Compression ratio \(=6 ;\) B.P. \(=14.8 \mathrm{~kW}\); Suction pressure \(=1\) bar and temp. \(=27^{\circ} \mathrm{C}\); Relative...
-
The following transactions were completed by Clark Management Company during the current fiscal year ended December 31: July 5. Received 70% of the $21,000 balance owed by Dockins Co., a bankrupt...
-
Apex Limited (APL) is a management consultancy company headquartered in Singapore. After the successful implementation of a share option plan for the senior management team in 20x1, the company has...
-
Complete and balance the equations below, and classify them as precipitation, acidbase, gasforming, or oxidationreduction reactions. Show states for reactants and products (s, , g, aq). (a) NiCO3 +...
-
Classify each of the following reactions as a precipitation, acidbase, or gas-forming reaction. Show states for the products (s, , g, aq), and then balance the completed equation. Write the net ionic...
-
a. Should you consider taking a position in U.S. bond index futures to hedge your investment in U.S. bonds? Explain. b. Should you consider taking a position in Japanese bond index futures to hedge...
-
Caleb, a high school wood and metal work teacher, has provided details of the following transactions during the year ended 3 0 June 2 0 2 3 : - Recieved salary of $ 1 0 4 , 0 0 0 for the school he is...
-
1. To what extent are entrepreneurial marketing and the strategic management process consistent? Inconsistent? Explain. 2. Discuss the relationship between market ownership, value networks, and the...
-
Big Data and Business Intelligence in Amazon (MO2-3) Amazon, one of the world's leading e-commerce platforms, serves millions of customers each day. The amount of data generated from customers'...
-
3. Use Excel to determine the probability of liquidity and illiquidity associated with the given values. 2 3.15 2.25 1.85 1.6 1 0.5 0.25 0 Probability of Liquidity Probability of Illiquidity
-
Coronado Industries has 26000 units in beginning finished goods. If sales are expected to be 140000 units for the year and Coronado desires an ending finished goods of 35000 units, how many units...
-
Hilton Corporation sold a press to its 80%-owned subsidiary, Agri Fab Inc., for $5,000 on January 1, 2012. The press originally was purchased by Hilton on January 1, 2011, for $20,000, and $6,000 of...
-
A glass manufacturer produces hand mirrors. Each mirror is supposed to meet company standards for such things as glass thickness, ability to reflect, size of handle, quality of glass, color of...
-
Assign a name for each of the following compounds. Be sure to assign the configuration of each chirality center and indicate the configuration(s) at the beginning of the name. a. b. c. Me
-
Identify the reactants you would use to form a racemic mixture of each of the following epoxides: a. b. c. d. Mery Me
-
Assume that the equation of state for a gas can be written in the form P(V m b(T)) = RT. Derive an expression for = 1/V (V /T)P and = 1/V (V /P)T for such a gas in terms of b(T), db(T)/dT, P, and...
-
Forensic Psychology's Role in the Legal SystemResources PSYC Discussion Participation Scoring Guide . In this unit, you were introduced to forensic psychology as a subfield of psychology as well as...
-
The nutrition label on a bottle of cola reports that a 8.0 fl oz serving contains 34g of sugar. How many kilograms of sugar are in a 2.00L bottle of cola? Use the conversion 1 L = 33.8 fl oz.
-
The bill and memo: Read A.3252 of 2023, The Billionaire Mark-to-Market Tax Act, and the accompanying bill memo. Bill memos are not always faithful to the bill so be sure to read the bill carefully....

Study smarter with the SolutionInn App


