Balance the following equations: (a) For the synthesis of urea, a common fertilizer (b) For the reactions
Question:
Balance the following equations:
(a) For the synthesis of urea, a common fertilizer
![]()
(b) For the reactions used to make uranium(VI) fluoride for the enrichment of natural uranium 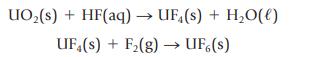
(c) For the reaction to make titanium(IV) chloride, which is then converted to titanium metal 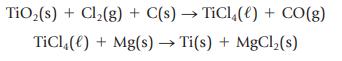
Transcribed Image Text:
CO,(g) + NH,(g) → NH,CONH,(s) + H,O({)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Lets balance the chemical equations a For the synthesis of urea COg 2NH3g NH2CONH2s H2O Explanation ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Balance the following equations using the method outlined in Section 3.7: (a) C + O2 CO (b) CO + O2 CO2 (c) C1 + Br2 HBr (d) K + C1O KOH + C1 (e) Mg + O2 MgO (f) O3 O2 (g) C1O2 C1O + O2 (h) N2 + C1...
-
Balance the following equations and write the corresponding ionic and net ionic equations (if appropriate): (a) (b) (c) Ba(OH)-(aq) + HPO 4 (aq )- HCIO4 (aq) + Mg(OH )2 (s)
-
Balance the following equations and write the corresponding ionic and net ionic equations (if appropriate): (a) (b) (c) CH3COOH (aq) + KOH(aq)- .co.(aq) + NaO H (aq) - HNO3(aq ) + Ba(OH)2(aq )-
-
Which one the below does not define "Work role boundaries" of a care worker limits that allow a patient and staff to connect safely in a therapeutic relationship based on patients' needs rules of...
-
Does our discussion of moneys usefulness as a medium of exchange and unit of account suggest reasons why some currencies become vehicle currencies for foreign exchange transactions?
-
From the U.S. Census Bureau document America's Families and Living Arrangements and an article in Time magazine, we found that, in 1963, 83.0% of American women between the ages of 25 and 54 were...
-
Name the various components of an I.C. engine.
-
The G. Company's financing plans for next year include the sale of long-term bonds with a 12 percent coupon. The company believes it can sell the bonds at a price that will give a yield to maturity...
-
Discuss anything you learned about personal financial management in Kindergarten through 12th grade. What do you remember? Has anyone in your life discussed financial management with you? If so, what...
-
Balance the following equations: (a) For the reaction to produce superphosphate fertilizer (b) For the reaction to produce diborane, B 2 H 6 (c) For the reaction to produce tungsten metal from...
-
In the following reactions, decide which reactant is oxidized and which is reduced. Designate the oxidizing agent and the reducing agent. (a) CH4(g) + 3 O(g) 2 CO(g) + 2 HO(l) (b) Si(s) + 2 Cl(g) ...
-
1. Why do you suppose that firms in Arctic nations are already developing specialized tanker ships and platforms for use in privately accessible Arctic areas? 2. What are possible opportunity costs...
-
What role does supplier relationship management play in fostering strategic partnerships and collaborative innovation within supply networks, particularly in industries characterized by rapid...
-
Evaluate the four traits of organizational learning and provide examples of how learning and change can impact one another. How important do you think is the four traits of organizational learning?
-
You work for a full -service marketing research firm based in downtown Ottawa that does focus groups using Recollective, online surveys with a panel of 100,000 people, and telephone surveys with a...
-
What measures can be implemented to address the growing cybersecurity threats targeting supply chain infrastructure, safeguarding critical assets and sensitive information against data breaches and...
-
What you learned in Advance program management class. How will this information help you determine if program management is a concept your organization will adopt? Do you believe this will be a...
-
Assume the same facts as in Exercise, but in addition, assume that Saratoga is itself in need of cash. It discounts the note received from Windsor at First Bank on July 1, 2013, at a discount rate of...
-
Which, if any, of the dichloroethene molecules drawn in Data Table II (3.) (4.) and (5.) are geometric isomers? A. B. C. D. cis-1,2-dichloroethene and trans-1,2-dichloroethene...
-
Propose a mechanism for the following transformation. Et Me [H,SO] Etw Me -
-
What reagents would you use to prepare each of the following thiols: a. b. c. SH SH
-
Predict the products for each of the following reactions. a. b. c. d. SH 1) NaOH Br 2) Br SNa
-
Dr. Tepper (Employee), a veterinarian licensed in Texas, enters into an Employment contract with Dr. Meng. Also, a licensed veterinarian, Dr. Meng (Employer) owns and operates the Barking Pet Clinic...
-
This course is UNDC201, it includes concepts, definitions, legal provisions, different types and methods as well as markets for organized criminal activities of organized criminal groups....
-
Do you agree with the statement "Conflict is an opportunity to turn a new leaf"? Why or Why not?

Study smarter with the SolutionInn App


