In the following reactions, decide which reactant is oxidized and which is reduced. Designate the oxidizing agent
Question:
In the following reactions, decide which reactant is oxidized and which is reduced. Designate the oxidizing agent and the reducing agent.
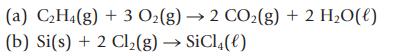
Transcribed Image Text:
(a) C₂H4(g) + 3 O₂(g) → 2 CO₂(g) + 2 H₂O(l) (b) Si(s) + 2 Cl₂(g) → SiCl4(e)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
In order to determine which reactant is oxidized and which is reduced in each of the given reactions ...View the full answer

Answered By

Mwangi Clement
I am a tried and tested custom essay writer with over five years of excellent essay writing. In my years as a custom essay writer, I have completed more than 2,000 custom essays in a diverse set of subjects. When you order essays from me, you are working with one of the best paper writers on the web. One of the most common questions I get from customers is: “can you write my essay?” Upon hearing that request, my goal is to provide the best essays and overall essay help available on the web. I have worked on papers in subjects such as Nursing and Healthcare, English Literature, Sociology, Philosophy, Psychology, Education, Religious Studies, Business, Biological Sciences, Communications and Media, Physical Sciences, Marketing and many others. In these fields, my specialties lie in crafting professional standard custom writings. These include, but are not limited to: research papers, coursework, assignments, term papers, capstone papers, reviews, summaries, critiques, proofreading and editing, and any other college essays.
My extensive custom writings experience has equipped me with a set of skills, research abilities and a broad knowledge base that allows me to navigate diverse paper requirements while keeping my promise of quality. Furthermore, I have also garnered excellent mastery of paper formatting, grammar, and other relevant elements. When a customer asks me to write their essay, I will do my best to provide the best essay writing service possible. I have satisfactorily offered my essay writing services for High School, Diploma, Bachelors, Masters and Ph.D. clients.
I believe quality, affordability, flexibility, and punctuality are the principal reasons as to why I have risen among the best writers on this platform. I deliver 100% original papers that pass all plagiarism check tests (Turnitin, Copyscape, etc.). My rates for all papers are relatively affordable to ensure my clients get quality essay writing services at reasonable prices.
4.50+
5+ Reviews
14+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
In the following reactions, decide which reactant is oxidized and which is reduced. Designate the oxidizing agent and the reducing agent. 2+ (a) CrO (aq) + 3 Sn+ (aq) + 14 H3O+ (aq) 2 Cr+ (aq) + 3...
-
1. Describe briefly how computers work and become tools for committing cyber-related crimes. 2. Use of proxies and VPNs is one of the methods cybercriminals try to cover their digital track and...
-
What is the short-run effect on the exchange rate of an increase in domestic real GNP, given expectations about future exchange rates?
-
Margin of error = 0.02; confidence level = 90%; likely range = 0.2 or less We have specified a margin of error, a confidence level, and a likely range for the observed value of the sample proportion....
-
Explain with neat sketches, the working of two-stroke diesel engine.
-
The grantor, G, was an 80% partner in a partnership. G lent the partnership money for construction financing and received a promissory note on market terms. G conveyed the note to a 10-year...
-
Explain one way compensation professionals can strengthen the pay-for-performance link? What the strengths of this way? And what are the drawbacks?
-
Balance the following equations: (a) For the synthesis of urea, a common fertilizer (b) For the reactions used to make uranium(VI) fluoride for the enrichment of natural uranium (c) For the reaction...
-
Which two of the following reactions are oxidation reduction reactions? Explain your answer briefly. Classify the remaining reaction. (a) CdCl(aq) + NaS(aq) CdS(s) + 2 NaCl(aq) (b) 2 Ca(s) + O(g) 2...
-
List and describe the key areas of concern for risk management.
-
What ethical considerations arise in global supply chain management, particularly regarding labor practices, human rights, and environmental stewardship, and how can responsible sourcing initiatives...
-
A builder of new homes in North Carolina has offered a $ 1 , 0 0 0 1 , 0 0 0 incentive bonus to any licensed broker whose customer or client purchases one of the buildings. The licensee that is...
-
What methodologies and metrics are employed to assess the total cost of ownership (TCO) across end-to-end supply chains, considering factors such as transportation costs, inventory carrying costs,...
-
The types of power used by a law enforcement organization can affect to what extent officers will comply with organizational rules and regulations. The paramilitary power associated with traditional...
-
How do change agents utilize network analysis techniques to identify informal power structures and influence networks within organizations, facilitating the diffusion of innovation and driving...
-
Saratoga Company owns 80% of the outstanding common stock of Windsor Company. On May 1, 2013, Windsor Company arranges a 1-year, $50,000 loan from Saratoga Company. The loan agreement specifies that...
-
Find the equation of the plane passing through the points P 5,4,3 ,Q 4,3,1 and R 1,5,4
-
Propose a plausible synthesis for each of the following transformations. a. b. c. d. e. CN En
-
Ethylene glycol is one of the main components of automobile antifreeze. Using iodoethane as your starting material, show how you could prepare ethylene glycol.
-
Propose a plausible synthesis for each of the following transformations. a. b. c. d. e. f. g. h. i. Br Br
-
1: In which case concerning the freedom of speech did Justice Oliver Wendell Holmes famously argue that the First Amendment wouldn't protect somebody yelling "fire" in a crowded theater? 2: When...
-
How does supercritical fluid extraction differ from traditional solvent-based extraction in terms of selectivity, efficiency, and environmental impact? Discuss the underlying thermodynamics of...
-
In preparing its bank reconciliation for the month of April 2025, Sheridan, Inc. has the following information available: Balance per bank statement, 4/30/25 $102820 NSF check returned with 4/30/25...

Study smarter with the SolutionInn App


