Calculate r H for the reaction 2 C(s) + 3 H(g) + /2O(g) given the information
Question:
Calculate ∆rH° for the reaction 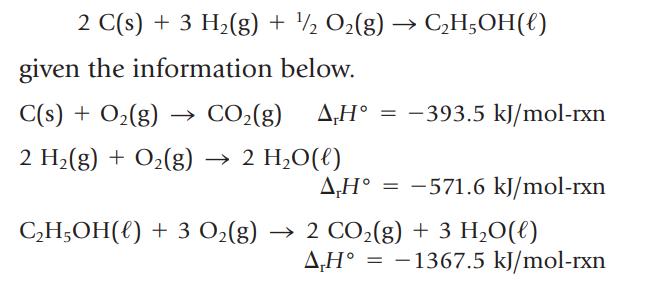
Transcribed Image Text:
2 C(s) + 3 H₂(g) + ¹/2O₂(g) given the information below. C(s) + 0₂(g) → CO₂(g) A,Hº = -393.5 kJ/mol-rxn 2 H₂(g) + O₂(g) → 2 H₂O(l) C₂H5OH() A,Hᵒ = -571.6 kJ/mol-rxn C₂H5OH() + 3 O₂(g) → 2 CO₂(g) + 3 H₂O(l) AH° -1367.5 kJ/mol-rxn =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
To calculate the standard enthalpy change rH for the reaction 2 Cs 3 H2g O2g C2H5OHl you need to use ...View the full answer

Answered By

Joram mutua
I am that writer who gives his best for my student/client. Anything i do, i give my best. I have tutored for the last five years and non of my student has ever failed, they all come back thanking me for the best grades. I have a degree in economics, but i have written academic papers for various disciplines due to top-notch research Skills.In additional, I am a professional copywriter and proofreader.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
The Internet has been a major disruptive force in modern retailing, helping bring about the demise of numerous familiar stores in your local mall: The Limited, American Apparel, Wet Seal,...
-
Iodobenzene dichloride, formed by the reaction of iodobenzene and chlorine, is a reagent for the chlorination of alkane C-H bonds. Chlorinations in which iodobenzene dichloride is used are initiated...
-
1. RHs CEO believes that the internet is limited in its ability to facilitate differentiation among retailers. Do you agree? Which retailers do a particularly effective job at presenting their...
-
A semicircular plate of radius r, oriented as in the figure, is submerged in fluid of density 68 lb/ft 3 so that its diameter is located at a depth of m feet. Calculate the force on one side of the...
-
Consider the following LP problem: (a) Convert these constraints to equalities by adding the appropriate slack, surplus, or artificial variables. Also, add the new variables into the problems...
-
Bryant Ltd. factors receivables with a carrying amount of 200,000 to Warren Company for 190,000 and guarantees all credit losses. Instructions Prepare the appropriate journal entry to record this...
-
The simplified financial statements of SPS Ltd appear below. Additional information 1. Dividends declared and paid were \($26\) 400. 2. During the year equipment was sold for \($10\) 200 cash. The...
-
Parker Tool is considering lengthening its credit period from 30 to 60 days. All customers will continue to pay on the net date. The firm currently bills $450,000 for sales and has $345,000 in...
-
X 2) Determine the vector moment, M, (in units of ft lbs) on the 8.5 foot beam about the origin as a result of the force F = 250 i + 175 j - 150 k (lbs) acting on it as shown. N 35 y F
-
You have the six pieces of metal listed below, plus a beaker of water containing 3.00 10 2 g of water. The water temperature is 21.00C. (a) In your first experiment you select one piece of metal and...
-
A piece of gold (10.0 g, C Au = 0.129 J/g K) is heated to 100.0C. A piece of copper (also 10.0 g, C Cu = 0.385 J/g K) is chilled in an ice bath to 0C. Both pieces of metal are placed in a beaker...
-
Show & Sell can advertise its products on local radio and television (TV), or in newspapers. The advertising budget is limited to $10,000 a month. Each minute of advertising on radio costs $15 and...
-
Patterson Development sometimes sells property on an installment basis. In those cases, Patterson reports income in its income statement in the year of the sale but reports installment income by the...
-
The president was the principal promoter and president of a corporation. He paid for the shares he had subscribed for. The corporation became insolvent and made an assignment for the benefit of its...
-
Question Content Area Net Present Value Carsen Sorensen, controller of Thayn Company, just received the following data associated with production of a new product: Expected annual revenues: $740,000...
-
8. An airplane is flying N75E with an airspeed of 550 mph and a 45 mph wind blowing S75E. What is the actual speed, in mph and direction, written as a bearing, of the plane? Round to the nearest 1...
-
Krystal suggests that the tax code allowing for a stepped - up basis ( a code section that allows heirs to receive inherited property with a basis that equals FMV at the time of death ) is flawed and...
-
Party Pools LLC assembles five different models of pool pumps for residential in-ground swimming pools. Fixed costs to produce model P07 pump are $75,000 per year. Variable costs per pump for this...
-
Place a tick in the appropriate grid to identify the balance that would be brought down in each of the following named accounts, in the books of Rizwy Mohamed: (a) In the Cash account: if Rizwy...
-
Predict the products of each of the following reactions: a. b. c. d. e. f. g. h. i. 1) BH3 THF 2) H2O2, NaOH Pt
-
The rate at which two methyl radicals couple to form ethane is significantly faster than the rate at which two tert-butyl radicals couple. Offer two explanations for this observation.
-
There are only two stereo-isomers of 1, 4-dimethylcyclohexane. Draw them, and explain why only two stereo-isomers are observed.
-
Part A A parallel-plate capacitor is formed from two 4.0 cm x4.0 cm electrodes spaced 2.9 mm apart. The electric field strength inside the capacitor is 1.0 106 N/C. What is the charge (in nC) on the...
-
A light, inextensible cord passes over a light, frictionless pulley with a radius of 13 cm. It has a(n) 21 kg mass on the left and a(n) 4.3 kg mass on the right, both hang- ing freely. Initially...
-
International trade has gone through many changes, especially during the last 60 years. It is really amazing how we can identify very different trends and dramatic changes in the way we interact...

Study smarter with the SolutionInn App


