You have the six pieces of metal listed below, plus a beaker of water containing 3.00
Question:
You have the six pieces of metal listed below, plus a beaker of water containing 3.00 × 102 g of water. The water temperature is 21.00°C.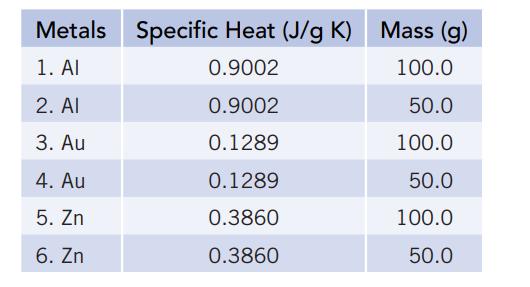
(a) In your first experiment you select one piece of metal and heat it to 100°C, and then select a second piece of metal and cool it to −10°C. Both pieces of metal are then placed in the beaker of water and the temperatures equilibrated. You want to select two pieces of metal to use, such that the final temperature of the water is as high as possible. What piece of metal will you heat? What piece of metal will you cool? What is the final temperature of the water?
(b) The second experiment is done in the same way as the first. However, your goal now is to cause the temperature to change the least, that is, the final temperature should be as near to 21.00°C as possible. What piece of metal will you heat? What piece of metal will you cool? What is the final temperature of the water?
Step by Step Answer:

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel





