Classify each of the following reactions as a precipitation, acidbase, or gas-forming reaction. Show states for the
Question:
Classify each of the following reactions as a precipitation, acid–base, or gas-forming reaction. Show states for the products (s, ℓ, g, aq), and then balance the completed equation. Write the net ionic equation.
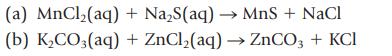
Transcribed Image Text:
(a) MnCl₂(aq) (b) K₂CO3(aq) + Na₂S(aq) → MnS + NaCl + ZnCl₂(aq) → ZnCO3 + KCI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
a MnCl2aq Na2Saq MnSs 2NaClaq This reaction is a precipitation reaction MnS is an insoluble solid pr...View the full answer

Answered By

Larlyu mosoti
I am a professional writer willing to do several tasks free from plagiarism, grammatical errors and submit them in time. I love to do academic writing and client satisfaction is my priority. I am skilled in writing formats APA, MLA, Chicago, and Harvard I am a statistics scientist and I can help out in analyzing your data. I am okay with SPSS, EVIEWS, MS excel, and STATA data analyzing tools.
Statistical techniques: I can do linear regression, time series analysis, logistic regression, and some basic statistical calculations like probability distributions. . I'm ready for your working projects!
Services I would offer:
• Academic writing.
• Article writing.
• Data entry.
• PDF conversion.
• Word conversion
• Proofreading.
• Rewriting.
• Data analyzing.
The best reason to hire me:
- Professional and Unique work in writing.
- 100% satisfaction Guaranteed
- within required time Express delivery
- My work is plagiarism Free
- Great communication
My passion is to write vibrantly with dedication. I am loyal and confident to give my support to every client. Because Client satisfaction is much more important to me than the payment amount. A healthy client-contractor relationship benefits in the longer term. Simply inbox me if you want clean work.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Classify each of the following reactions as a precipitation, acidbase, or gas-forming reaction. Show states for the products (s, , g, aq), and then balance the completed equation. Write the net ionic...
-
Classify each of the following reactions as one of the four possible types summarized in Table 19.3: (a) (b) (c) N2(g) 3 F2(g)2NF3(g) AH249 kJ; AS278 J/K N2(g) + 3C12(g) --> 2NC3(g) AH 460 kJ; AS...
-
The figure below represents a schematic of pipe network. A rate of 35 Ls, is pumped to feed two lines (3-4-5-6; and 2-7-8). The length and diameter of each pipe segment are listed in the table....
-
Between 1984 and 1985, the money supply in the United States increased to $641.0billion from $570.3billion, while that of Brazil increased to 106.1 billion cruzados from 24.4 billion. Over the same...
-
Alligators perform a spinning maneuver, referred to as a "death roll", to subdue their prey. Videos were taken of juvenile alligators performing this maneuver in a study for the article "Death Roll...
-
A four stroke six cylinder engine with stroke volume of 1.75 litres develops \(26.25 \mathrm{~kW}\). The mean effective pressure is 6 bar. Find speed of the engine.
-
A Nielsen study indicates that 18 to 24 year old spend a mean of 135 minutes watching video on their smartphones per month. (Data extracted bit. ly/1hF3BP2.) Assume that the amount of time watching...
-
Match each definition with its related term by selecting the appropriate term in the dropdown provided. 1. Actual Accounting System Term 2. Direct Labor Efficiency Variance 3. Direct Labor Rate...
-
Balance each of the following equations, and classify them as precipitation, acidbase, gas-forming, or oxidationreduction reactions. Show states for reactants and products (s, , g, aq). (a) CuCl + HS...
-
Give a formula for each of the following compounds: (a) A soluble compound containing the bromide ion (b) An insoluble hydroxide (c) An insoluble carbonate (d) A soluble nitrate-containing compound...
-
Hero Manufacturing has 7.6 million shares of common stock outstanding. The current share price is $67 and the book value per share is $4. The company also has two bond issues outstanding, both with...
-
Sweet Treats Beverage Company's operating activities for the year are listed below. Purchases Operating expenses Beginning inventory Ending inventory Sales revenue $140,600 80,800 12,400 18,100...
-
Strategic marketing consists of many key elements but out of those the most important key element is the value proposition. This is used to show the value of the product that is being marketed to the...
-
How has technology allowed banks to expand their marketing channels beyond branches? Could you describe four (4) technology-related distribution channels for banking services. Also please analyze...
-
What role does emotional intelligence play in conflict management, and how can emotional regulation techniques enhance conflict resolution effectiveness in interpersonal and organizational contexts ?
-
What technology could the executive director use to improve marketing efforts? Which social media channels are the ones she would find most success with, and why?
-
On January 1, 2012, Jungle Company sold a machine to Safari Company for $30,000. The machine had an original cost of $24,000, and accumulated depreciation on the asset was $9,000 at the time of the...
-
The Home Depot is the leading retailer in the home improvement industry and one of the 10largest retailers in the United States. The company included the following on its January 29, 2012, balance...
-
Benzoic acid, 1.35 g, is reacted with oxygen in a constant volume calorimeter to form H 2 O(l) and CO 2 (g) at 298 K. The mass of the water in the inner bath is 1.55 10 3 g. The temperature of the...
-
Calculate the P and T values for which Br2(g) is in a corresponding state to Xe(g) at 330. K and 72.0 bar.
-
For each of the following reactions, predict the product and draw the mechanism of its formation. a. b. c. d. e. f. 1) PhMgBr 2) H20 Me 1) NaCN 2) H20 *Me
-
Police officers are called to the house at 268 South Main Street after a neighbor notices newspapers piling up at the front door. The house is occupied by Carl, his wife Becca, and two small...
-
Derby State University is a four-year public institution of higher education that features a robust and award-winning STEM curriculum. Located in the Southern United States, the heritage of...
-
Moving Up Chemical Productions Pty Ltd ('the company') holds an environment protection licence ('EPL') under the Protection of the Environment Operations Act 1997 (NSW) ('the POEO Act') in relation a...

Study smarter with the SolutionInn App


