Consider the following half-reactions: (a) Based on E values, which metal is the most easily oxidized? (b)
Question:
Consider the following half-reactions: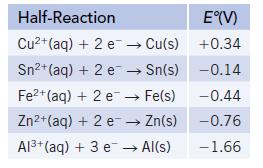
(a) Based on E° values, which metal is the most easily oxidized?
(b) Which metals on this list are capable of reducing Fe2+(aq) to Fe(s)?
(c) Write a balanced chemical equation for the reaction of Fe2+(aq) with Sn(s). Is this reaction product-favored or reactant-favored at equilibrium?
(d) Write a balanced chemical equation for the reaction of Zn2+(aq) with Sn(s). Is this reaction product-favored or reactant-favored at equilibrium?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





