Europium has two stable isotopes, 151 Eu and 153 Eu, with masses of 150.9197 u and 152.9212
Question:
Europium has two stable isotopes, 151Eu and 153Eu, with masses of 150.9197 u and 152.9212 u, respectively. Calculate the percent abundances of these isotopes of europium.

Transcribed Image Text:
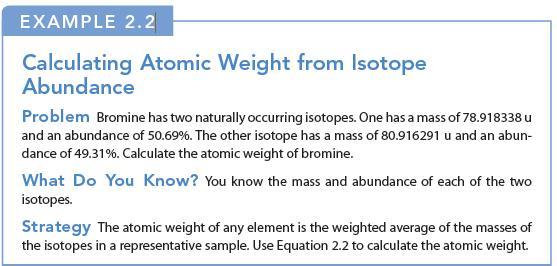
EXAMPLE 2.2 Calculating Atomic Weight from Isotope Abundance Problem Bromine has two naturally occurring isotopes. One has a mass of 78.918338 u and an abundance of 50.69%. The other isotope has a mass of 80.916291 u and an abun- dance of 49.31%. Calculate the atomic weight of bromine. What Do You Know? You know the mass and abundance of each of the two isotopes. Strategy The atomic weight of any element is the weighted average of the masses of the isotopes in a representative sample. Use Equation 2.2 to calculate the atomic weight.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
To calculate the percent abundances of the two stable isotopes of europium Eu you can use the same m...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Gallium has two naturally occurring isotopes, 69 Ga and 71 Ga, with masses of 68.9257 u and 70.9249 u, respectively. Calculate the percent abundances of these isotopes of gallium. EXAMPLE 2.2...
-
Money market securities are characterized by: I. Maturity less than 1 year II. Safety of the principal investment III. Low rates of return O O O O I and III only I, II, and III I and II only I only
-
The element europium exists in nature as two isotopes: 151Eu has a mass of 150.9196 amu, and 153Eu has a mass of 152.9209 amu. The average atomic mass of europium is 151.96 amu. a. Calculate the...
-
Deuterium ( ) is an attractive fuel for fusion reactions because it is abundant in the oceans, where about 0.015% of the hydrogen atoms in the water (H 2 O) are deuterium atoms. (a) How many...
-
List and describe various types of output, including technology-based forms of information delivery.
-
Todd's Direct, a major TV sales chain headquartered in New Orleans, is about to open its first outlet in Mobile, Alabama, and wants to select a site that will place the new outlet in the center of...
-
Differentiate technology from methodology and from method. Can you come up with an example that differentiates these concepts in a specific context, perhaps software development?
-
When a company acts in an ethically questionable manner, what types of problems are caused for the organization and its customers?
-
Make scientific discussion and conclusion for a poster about bladeless wind turbines using the following information. Bladeless wind turbines have several promising applications due to their unique...
-
Titanium and thallium have symbols that are easily confused with each other. Give the symbol, atomic number, atomic weight, and group and period number of each element. Are they metals, metalloids,...
-
Verify that the atomic weight of magnesium is 24.31, given the following information: 24 Mg, mass = 23.985042 u; percent abundance = 78.99% 25 Mg, mass = 24.985837 u; percent abundance = 10.00% 26...
-
Think about your typical day. Do you (and your team) spend most of your time reacting to problems and your boss requests and seeking to control others? Or do you spend most of your time pursuing your...
-
discuss how the different areas of the brain, specifically Broca's and Wernicke's areas, contribute to forming, producing, and understanding language. Also, discuss how brain functions differ...
-
6. What is wrong in the following code? public class Test ( a. b. c. d. public static void main(String[] args) { methodl (); } private static void methodl () { Circle c; System.out.println ("What is...
-
[3] Explain why the following assembly language and RTL constructs are incorrect. a. D3, #4 b. [D3], D2 (D3), D2 C. d. e. f. MOVE MOVE MOVE [D3] [D3] 3 [D3] A0 + 3 #3
-
5. Let X be a kn x n matrix and Y by an n x kn matrix, for some integer k. (a) Describe an algorithm that computes the product XY using Strassen's algorithm as a subroutine, i.e., use it as a...
-
6. The standard algorithm for multiplying two n-bit binary integers r and y costs (n). A naive divide-and-conquer algorithm is to let z=: 2/2a +b and y = 2n/c+d, then ry = = (2/2a + b) (2/2c+d) 2" ac...
-
Southcenter Variety Store has many accounts receivable. The Southcenter balance sheet, December 31, 20X1 showed Accounts Receivable, $950,000, and Allowance for Uncollectible Accounts, $40,000. In...
-
The polar coordinates of a point are given. Find the rectangular coordinates of the point. (-1, - /3)
-
Calculate q, w, U, and H if 2.25 mol of an ideal gas with C v,m = 3/2 R undergoes a reversible adiabatic expansion from an initial volume V i = 5.50 m 3 to a final volume. V f =25.0 m 3 .The initial...
-
For an ideal gas, (U/V) T and (h/P) T = 0. Prove that C V is independent of volume and C P is independent of pressure.
-
Assume the internal energy of an elastic fiber under tension (see Problem 6.16) is given by. dU = T dS P dV F dl. Obtain an expression for, (g/L) P,T and calculate the maximum non-expansion work...
-
On 1st January, 2015, Vinod drew and Pramod accepted a bill at three months for ~ 2,000. On 4th January, 2015 Vinod discounted the bill with his bank at 15% p.a. and remitted half the proceeds to...
-
On February 9, 1996, Walt Disney Co. acquired Capital Cities/ ABC Inc. for $10.1 billion in cash and 155 million shares of Disney valued at $8.8 billion, based on the stock price at the date the...
-
171.A copy machine acquired on March 1, 2011 with a cost of $1,410 has an estimated useful life of 3 years. Assuming that it will have a residual value of $150, determine the depreciation for the...

Study smarter with the SolutionInn App


