Verify that the atomic weight of magnesium is 24.31, given the following information: 24 Mg, mass =
Question:
Verify that the atomic weight of magnesium is 24.31, given the following information:
24Mg, mass = 23.985042 u; percent abundance = 78.99%
25Mg, mass = 24.985837 u; percent abundance = 10.00%
26Mg, mass = 25.982593 u; percent abundance = 11.01%

Transcribed Image Text:
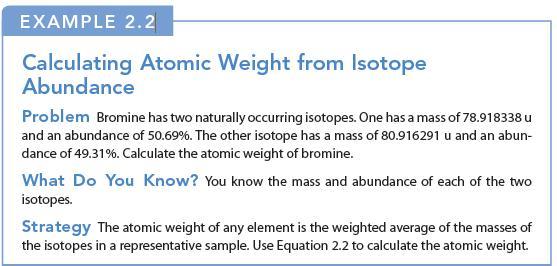
EXAMPLE 2.2 Calculating Atomic Weight from Isotope Abundance Problem Bromine has two naturally occurring isotopes. One has a mass of 78.918338 u and an abundance of 50.69%. The other isotope has a mass of 80.916291 u and an abun- dance of 49.31%. Calculate the atomic weight of bromine. What Do You Know? You know the mass and abundance of each of the two isotopes. Strategy The atomic weight of any element is the weighted average of the masses of the isotopes in a representative sample. Use Equation 2.2 to calculate the atomic weight.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
To calculate the atomic weight of magnesium Mg given the isotopic data pro...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Verify that the atomic weight of lithium is 6.94, given the following information: 6 Li, mass = 6.015121 u; percent abundance = 7.50% 7 Li, mass = 7.016003 u; percent abundance = 92.50% EXAMPLE 2.2...
-
Thallium has two stable isotopes, 203 Tl and 205 Tl. Knowing that the atomic weight of thallium is 204.4, which isotope is the more abundant of the two? EXAMPLE 2.2 Calculating Atomic Weight from...
-
Europium has two stable isotopes, 151 Eu and 153 Eu, with masses of 150.9197 u and 152.9212 u, respectively. Calculate the percent abundances of these isotopes of europium. EXAMPLE 2.2 Calculating...
-
The Home Depot, Inc. (HD) operates over 2,200 home improvement retail stores and is a competitor of Lowe's (LOW). The following data (in millions) were adapted from recent financial statements of The...
-
Describe six types of user interface controls, and provide an example of how you could use each type in a data entry screen.
-
Courtney Kamauf schedules production of a popular Rustic Coffee Table at Kamauf Enterprises, Inc. The table requires top, four legs, 1/8 gallon of stain, 1/16 gallon of glue, 2 short braces between...
-
What is the difference between object-oriented languages and UML?
-
1. Wages and prices will increase when actual output exceeds potential. __________(True/False) 2. The short run in macroeconomics is the time period over which __________do not adjust to economic...
-
A scientific instrument that accurately measures the strength of adhesives (sticky tape). -Tape Adhesion strength testing . How it works: The stepper motor drives the probe vertically down and...
-
Gallium has two naturally occurring isotopes, 69 Ga and 71 Ga, with masses of 68.9257 u and 70.9249 u, respectively. Calculate the percent abundances of these isotopes of gallium. EXAMPLE 2.2...
-
Name and describe the composition of the three hydrogen isotopes.
-
A standard air conditioner involves a refrigerant that is typically now a fluorinated hydrocarbon, such as CH2F2. An air-conditioner refrigerant has the property that it readily vaporizes at...
-
3.2. Generate thirty points (x,y;), i = 1,2,..., 30, by the MATLAB code randn ('seed', 314); x=linspace(0, 1,30)'; y=2*x.^2-3 *x+1+0.05*randn (size (x)); Find the quadratic function y = ax +bx+c that...
-
fix this bug I am getting: I added all my code below OUTPUT: Team 1: Team Name: Tigers Owner: Bob Team 2: Team Name: Raiders Owner: Darrell //I think error is caused by not being able to read the...
-
There are four steps in risk management: Risk identification , is the careful and systematic discovery of all risks that confront a household or an organization. Risk measurement . Each risk can be...
-
What are some challenges for law enforcement when investigating international organized crime from a criminal balance intelligence perspective? please include any references
-
Sunshine Pharmaceuticals is a manufacturer engaged in the development and marketing of new drugs. The chief research chemist at Sunshine Pharmaceuticals, Dr. I. Smart, informed the president, Ms. T....
-
Prepare all journal entries for 20X2 concerning the following data for a medical clinic that performs elective laser surgery that corrects vision. Such procedures are not covered by third-party...
-
A survey of 70 college freshmen asked whether students planned to take biology, chemistry, or physics during their first year. Use the diagram to answer each question. How many of the surveyed...
-
Why do the z and y components of the velocity not change in the collision depicted in Figure 1.2? Figure 1.2 mvx mvx
-
A mixture of 2.10 10 3 g of O 2 , 3.88 10 -3 mol of N 2 , and 5.25 10 20 molecules of CO are placed into a vessel of volume 5.25 L at 12.5C. a. Calculate the total pressure in the vessel. b....
-
Propose a plausible synthesis for each of the following transformations: (a) (b) (c) (d)
-
What is the most important Linux command line tools are for Linux forensic evaluations? Why is it important? How is the tool used?
-
Who develops Kali Linux? What distribution was the predecessor to Kali Linux? 2. What is the main purpose(s) of the Kali Linux distribution? 3. What are the installation options for Kali Linux?...
-
What are the pros and cons of the Linux OS. List the following in your answer: Brief history of the Linux OS Different Linux OS versions What is open source verses closed source In what...

Study smarter with the SolutionInn App


