Gallium has two naturally occurring isotopes, 69 Ga and 71 Ga, with masses of 68.9257 u and
Question:
Gallium has two naturally occurring isotopes, 69Ga and 71Ga, with masses of 68.9257 u and 70.9249 u, respectively. Calculate the percent abundances of these isotopes of gallium.

Transcribed Image Text:
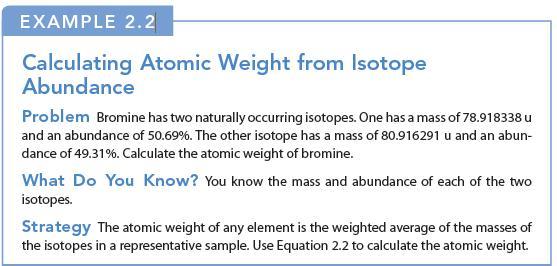
EXAMPLE 2.2 Calculating Atomic Weight from Isotope Abundance Problem Bromine has two naturally occurring isotopes. One has a mass of 78.918338 u and an abundance of 50.69%. The other isotope has a mass of 80.916291 u and an abun- dance of 49.31%. Calculate the atomic weight of bromine. What Do You Know? You know the mass and abundance of each of the two isotopes. Strategy The atomic weight of any element is the weighted average of the masses of the isotopes in a representative sample. Use Equation 2.2 to calculate the atomic weight.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
To calculate the percent abundances of the two naturally occurring isotopes of gallium 69Ga and 71Ga ...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Find the currents in the circuit given below. R1 = 1ohm,R2 = 4 ohm, R3 = 30hm, R4 = 6ohm,R5 = 12ohm, R6 = 4 ohm, R7 = 4hmR8 = 2hhmE = 12v
-
Europium has two stable isotopes, 151 Eu and 153 Eu, with masses of 150.9197 u and 152.9212 u, respectively. Calculate the percent abundances of these isotopes of europium. EXAMPLE 2.2 Calculating...
-
Gallium (Ga) consists of two naturally occurring isotopes with masses of 68.926 and 70.925 amu. (a) How many protons and neutrons are in the nucleus of each isotope? Write the complete atomic symbol...
-
Using the adjustment data listed in P3-2 for San Mateo Health Care, indicate the effects of each adjustment on the liquidity metric Quick Assets and profitability metric Net Income - Accrual Basis....
-
Define detail reports, exception reports, and summary reports. Explain the concept of a control field and how it is used to produce a control-break report.
-
A firm based in Mexico has found that its growth is restricted by the limited liquidity of the Mexican capital market. List the firm's options for raising money on the global capital market. Discuss...
-
Consider the ammonia process in which \(\mathrm{N}_{2}\) and \(\mathrm{H}_{2}\) (with impurities \(\mathrm{Ar}\) and \(\mathrm{CH}_{4}\) ) are converted to \(\mathrm{NH}_{3}\) at high pressure...
-
This problem continues the Draper Consulting, Inc., situation from Problem 18-37 of Chapter 18. Draper Consulting provides consulting service at an average price of $175 per hour and incurs variable...
-
Question 2 (50 marks) Compute the slope and deflection of the point B in the diagram below. (a) Use the moment-area method. [25 marks] (b) Use the conjugate beam method. [25 marks] Assume P= (1+0.Y)...
-
Titanium and thallium have symbols that are easily confused with each other. Give the symbol, atomic number, atomic weight, and group and period number of each element. Are they metals, metalloids,...
-
Verify that the atomic weight of magnesium is 24.31, given the following information: 24 Mg, mass = 23.985042 u; percent abundance = 78.99% 25 Mg, mass = 24.985837 u; percent abundance = 10.00% 26...
-
On January 1, 2019, Devlin Company (seller-lessee) sold heavy-duty equipment to Bancroft Bank (buyer-lessor) for its fair market value of $6,400,000 and immediately leased it back under a 20-year...
-
Objective: To develop a program to train executives in negotiation skills. Imagine that you are a partner of Brain Consulting, a consulting firm that offers programs to develop the soft skills of...
-
Identify a recent interpersonal, group, or organizational conflict that you were involved in and was later resolved. Select five of the topics listed below and discuss how they apply to your chosen...
-
The DE partnership is undergoing an installment liquidation. Partners D and E share income in a 3:2 ratio and have current capital balances of $63,000 and $90,000, respectively. No loans are...
-
. Discuss how is the top-down approach to information security superior to the bottom-up approach. Which approach was used in your organization? 2. Can recent college graduates expect to be project...
-
Identify an example of a situation or experience that is able to explain about the social contract ethic.
-
You have been asked to analyze a project where the analyst has estimated the return on capital to be 37% over the ten-year lifetime of the project. The cost of capital is only 12%, but you have...
-
A new car sold for $31,000. If the vehicle loses 15% of its value each year, how much will it be worth after 10 years?
-
Is H for breaking the first CH bond in methane equal to the average CH bond enthalpy in this molecule? Explain your answer.
-
Under anaerobic conditions, glucose is broken down in muscle tissue to form lactic acid according to the reaction: C 6 H 12 O 6 (s) 2CH 3 CHOHCOOH(aq). Thermodynamic data at T = 298 K for glucose and...
-
Which of the following systems are isolated? a) A bottle of wine b) A tightly sealed, perfectly insulated thermos bottle c) A tube of toothpaste d) our solar system. Explain your answers.
-
The premise of the experiment is: In a group of 10 people, present your findings with different mathematical models (eg, logistics, SI, SIS) or any other mathematical models. An initial person has a...
-
The rule of 72. 6. Show in Excel that if $20,000 is invested at 6% compounded annually, then it will double in about 12 years. (Note: 12=72/6) year F 1 23456780 9 Calculate the future value as the...
-
Using the following table, calculate the missing values for Jake's 15year student loan for $13,000.00. The annual interest rate is 7.288%, compounded each month. Find the data missing from the table...

Study smarter with the SolutionInn App


