For each of the following electrochemical cells, write equations for the oxidation and reduction half-reactions and for
Question:
For each of the following electrochemical cells, write equations for the oxidation and reduction half-reactions and for the overall reaction.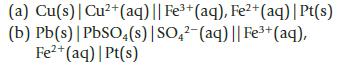
Transcribed Image Text:
(a) Cu(s) | Cu²+ (aq) || Fe³+ (aq), Fe²+ (aq) | Pt(s) (b) Pb(s) | PbSO4(s) | SO4²-(aq) || Fe³+ (aq), Fe²+ (aq) | Pt(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a Cus Cuaq Feaq Feaq Pts Oxidation HalfReaction at the anode Cus Cuaq 2e ...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
For each of the following electrochemical cells, write equations for the oxidation and reduction half-reactions and for the overall reaction. (a) Pb(s) | Pb+ (aq) || Sn4+ (aq), Sn+ (aq) | C(s) (b)...
-
Each of the following equations describes a reaction of a compound called methyl formate. To what class of compounds does methyl formate belong? Which reactions require a reducing agent? Which...
-
Write balanced equations for each of the following reactions (some of these are analogous to reactions shown in the chapter). (a) Aluminum metal reacts with acids to form hydrogen gas. (b) Steam...
-
The quarterly sales for a software product over the past three years are given in the table below. 1) Forecast the demand for year 4 using the moving average technique for 3 periods. 1) Compute the...
-
ERP software programs allow tighter linkages within a supply chain than were possible with earlier generations of software. Consider the possibility of a tighter link between the marketing and...
-
Match the organizations on the left with the functions on the right. Each function should be used only once. Organization Function Institute of Internal Auditors a. The group that creates and...
-
Three bars, each made of different materials, are connected together and placed between two walls when the temperature is T 1 = 12C. Determine the force exerted on the (rigid) supports when the...
-
Suppose that all of the checks issued to the defendants were made payable to Fasig-Tipton Co., Fasig-Tipton Midlantic, Inc. Under the Uniform Commercial Code, were the instruments payable jointly or...
-
What are the pros and cons of recruitment and selection in an Internet context? Provide examples of your personal experiences with online recruitment and selection. Do you agree or disagree with...
-
In Chapter 13, you learned that entropy, as well as enthalpy, plays a role in the solution process. If H for the solution process is zero, explain how the process can be driven by entropy.
-
Chloroacetic acid, ClCH 2 CO 2 H, is a moderately weak acid (K a = 1.40 10 3 ). If you dissolve 94.5 mg of the acid in water to give 125 mL of solution, what is the pH of the solution?
-
Boiling water in an aluminum pan is being converted to steam at a rate of 10.0 g/s. The flat bottom of the pan has an area of 325 cm2 and the pan's thickness is 3.00 mm. If 27.0% of all heat that is...
-
Responsibility in many of these areas is facilitated when sex takes place within the context of an ongoing relationship. In that sense, sexual responsibility is a matter of values. Is responsible sex...
-
If family or friends offer you advice or information about investing, finances, or business,? i. what are your professional qualifications? ii. where did your information come from? iii. are you...
-
Hyunwoo is a human resource professional working for an electronics firm. She is studying various research on group dynamics, and how it can provide the best working conditions for an employee. She...
-
In the study of human behaviors, organizational leaders must understand concepts such as values, attitudes, perceptions, and behaviors of people. The objective is to align their thoughts, emotions,...
-
It has been said that International Law remains a " contradiction in terms ." Discuss how International Law treats individual persons (i.e. human beings) compared to nation states ? Explain your...
-
In theory working for the government means that job security is greater than working for private enterprise. Are married people more likely to work for the government (WRKGOVT: 1 = Government, 2 =...
-
Explain why it is not wise to accept a null hypothesis.
-
For laser action to occur, the medium used must have at least three energy levels. What must the nature of each of these levels be?
-
If Plancks constant were smaller than it is, would quantum phenomena be more or less conspicuous than they are now?
-
In the Bohr model of the hydrogen atom, the radius of the electrons orbit in the ground state is 5.3 10 -11 m. What aspect of the quantum-mechanical model of this atom would you expect to correspond...
-
The Joker has set a trap for Batman, using his own son Nemo as bait. The Joker attaches a very heavy bowling ball of mass M= 300kg to a long wire of length =30m, then attaches the other end of the...
-
Describe the concept of persistent memory and how it differs from traditional volatile memory. What are the implications of persistent memory for system crash recovery, data consistency, and...
-
Imagine an apple, car, human, jet plan and peanut are sitting on the surface of the Earth. Which would exert the greatest gravitational pull on planet Earth? Put them in order from smallest...

Study smarter with the SolutionInn App


