In the discussion on the composition of air, mention is made of the fact that water vapor
Question:
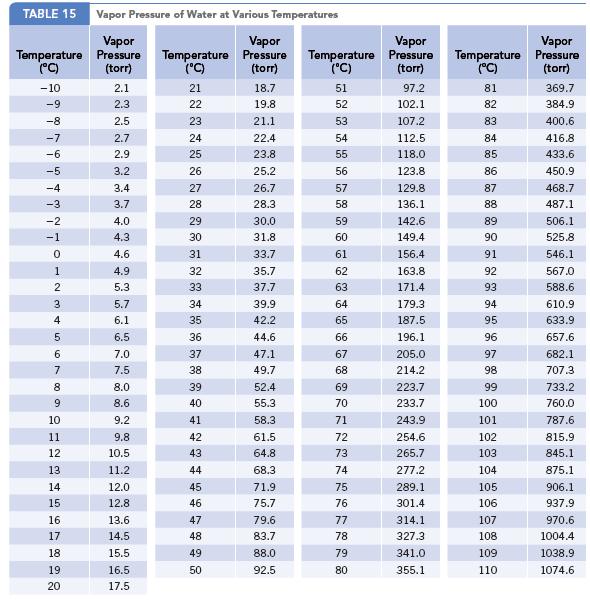
In the discussion on the composition of air, mention is made of the fact that water vapor may have a concentration as high as 40,000 ppm. Calculate the partial pressure exerted by water vapor at this concentration. Assume that this represents a situation with 100% humidity. What temperature would be needed to achieve this value? (See Appendix G.)
Data given in Appendix G
Transcribed Image Text:
TABLE 15 Vapor Pressure of Water at Various Temperatures Vapor Pressure (torr) Temperature (°C) -10 -9 -8 -7 -6 -5 -4 -3 -2 -1 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 2.1 2.3 2.5 2.7 2.9 3.2 3.4 3.7 4.0 4.3 4.6 4.9 5.3 5.7 6.1 6.5 7.0 7.5 8.0 8.6 9.2 9.8 10.5 11.2 12.0 12.8 13.6 14.5 15.5 16.5 17.5 Temperature (°C) 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 Vapor Pressure (torr) 18.7 19.8 21.1 22.4 23.8 25.2 26.7 28.3 30.0 31.8 33.7 35.7 37.7 39.9 42.2 44.6 47.1 49.7 52.4 55.3 58.3 61.5 64.8 68.3 71.9 75.7 79.6 83.7 88.0 92.5 Temperature (°C) 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 Vapor Pressure (torr) 97.2 102.1 107.2 112.5 118.0 123.8 129.8 136.1 142.6 149.4 156.4 163.8 171.4 179.3 187.5 196.1 205.0 214.2 223.7 233.7 243.9 254.6 265.7 277.2 289.1 301.4 314.1 327.3 341.0 355.1 Temperature (°C) 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108. 109 110 Vapor Pressure (torr) 369.7 384.9 400.6 416.8 433.6 450.9 468.7 487.1 506.1 525.8 546.1 567.0 588.6 610.9 633.9 657.6 682.1 707.3 733.2 760.0 787.6 815.9 845.1 875.1 906.1 937.9 970.6 1004.4 1038.9 1074.6
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
To calculate the partial pressure of water vapor at a concentration of 40000 ppm we can use the foll...View the full answer

Answered By

David Muchemi
I am a professional academic writer with considerable experience in writing business and economic related papers. I have been writing for my clients who reach out to me personally after being recommended to me by satisfied clients.
I have the English language prowess, no grammatical and spelling errors can be found in my work. I double-check for such mistakes before submitting my papers.
I deliver finished work within the stipulated time and without fail. I am a good researcher on any topic especially those perceived to be tough.
I am ready to work on your papers and ensure you receive the highest quality you are looking for. Please hire me to offer my readily available quality service.
Best regards,
4.60+
27+ Reviews
61+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
The first quarter of 2008 had not yet ended and Steve Savage already knew the company would surpass the projected $22 million in revenues for the year. He and the management team had doubled sales...
-
Software the Hard Way Joe is a software developer working for Ace Development Company for the last 10 years. He has no management position with the company other than being a software developer,...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
State Newtons second law of motion. What are the limitations on the use of Newtons second law? Explain.
-
Procter and Gamble sold a detergent, Ace con Blanqueador, in Puerto Rico. It advertised that "Mas blanco no se puede" (Whiter is not possible). Clorox, a bleach maker, sued Procter and Gamble...
-
Cal bought a 1952 Mickey Mantle Topps baseball card for $17,750 from Theodore, who represented that the card was in near-mint condition. Cal put the card in a safe-deposit box. Two years later, Cal...
-
Consider the following cash flow profile, and assume MARR is 10 percent/year and the finance rate is 4 percent/year. a. Determine the MIRR for this project. b. Is this project economically...
-
Goltra Clinic is considering investing in new heart-monitoring equipment. It has two options: Option A would have an initial lower cost but would require a significant expenditure for rebuilding...
-
How to convert this Entity-Relationship data model into a relational database model? Contains Inventory Inventory ID Customer Cust_ID Amount Availible Cust LName Must Have Coffee_Type Cust Fname...
-
Which of the following atmospheric gases is present in highest concentration in dry air? (a) N 2 O (b) CH 4 (c) O 3 (d) CO
-
A hydrogen-oxygen fuel cell operates on the simple reaction H 2 (g) + 1/2 O 2 (g) H 2 O() If the cell is designed to produce 1.5 A of current and if the hydrogen is contained in a 1.0-L tank at 200...
-
Propose a mechanism for conversion of the dianion to the ketone under mildly acidic conditions.
-
Steven, age 40 is expected to earn $55,000 as a Financial Advisor. What will Steven's 2020 CPP contribution be? Show your calculation
-
Assume that the Modigliani-Miller assumptions hold and we have no taxes. Petro Inc. has no debt; its unlevered equity consists of 100,000 shares trading at $65 each. The unlevered equity has a beta...
-
What earning management for exchange difference on translation of foreign operation is $66,501,000 and -$113,160,000 in 2022 and 2023, and the difference is $46,659,000 taking a big bath ?
-
The Log-normal Distribution. In atmospheric science, the Log-normal distribution is often used to characterize particle size distributions. In one study, the distribution of silicone nanoparticle...
-
LA Moving Company has the following data, dollars in thousands. If it follows the residual dividend model, what will its dividend payout ratio be? Capital budget % Debt Net income (NI) $5,900 40%...
-
Cutler Petroleum, Inc., is trying to evaluate a generation project with the following cash flows: Year Cash Flow_ 0 .....................-$ 85,000,000 1 ...................... 125,000,000 2...
-
Record the following selected transactions for March in a two-column journal, identifying each entry by letter: (a) Received $10,000 from Shirley Knowles, owner. (b) Purchased equipment for $35,000,...
-
A 500-nm lightwave in vacuum enters a glass plate of index 1.60 and propagates perpendicularly across it. How many waves span the glass if its 1.00 cm thick?
-
Yellow light from a sodium lamp ( 0 = 589 nm) traverses a tank of glycerin (of index 1.47), which is 20.0 m long, in a time t 1 . If it takes a time t 2 for the light to pass through the same tank...
-
A lightwave travels from point A to point B in vacuum. Suppose we introduce into its path a flat glass plate (n g = 1.50) of thickness L = 1.00 mm. If the vacuum wavelength is 500 nm, how many waves...
-
What is the best thing a programmer can do to improve memory access time? How does it improve memory access time?
-
consider a two - tier memory system consisting of cache (SRAM) and main memory (D RAM). The cache access time is 1 nsec and the main memory access time is 50 nsecs. (1 nsec = 1 10 - 9 secs). (a)...
-
A) What is the minimum number of memory accesses needed to access a location in virtual memory? For the following questions, assume we have a Translation Look-aside Buffer (TLB) and 2-level page...

Study smarter with the SolutionInn App


