In the following reactions, decide which reactant is oxidized and which is reduced. Designate the oxidizing agent
Question:
In the following reactions, decide which reactant is oxidized and which is reduced. Designate the oxidizing agent and the reducing agent.
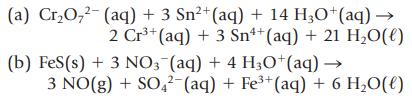
Transcribed Image Text:
2+ (a) Cr₂O₂² (aq) + 3 Sn²+ (aq) + 14 H3O+ (aq) → 2 Cr³+ (aq) + 3 Sn+ (aq) + 21 H₂O(l) (b) FeS (s) + 3 NO3(aq) + 4 H3O+ (aq) → 3 NO(g) + SO4² (aq) + Fe³+ (aq) + 6 H₂O(l)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To determine which reactant is oxidized and which is reduced in each of the given reactions you can ...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
In the following reactions, decide which reactant is oxidized and which is reduced. Designate the oxidizing agent and the reducing agent. (a) CH4(g) + 3 O(g) 2 CO(g) + 2 HO(l) (b) Si(s) + 2 Cl(g) ...
-
Using real employed samples, multiple field studies indicate that fat women experience more negative outcomes than fat men. In one study conducted by Steven Gortmaker and colleagues and using over...
-
Multinationals generally have production plants in a number of countries. Consequently, they can move production from expensive locations to cheaper ones in response to various economic developmentsa...
-
In the article "Happier and Less Isolated: Internet Use in Old Age" (Journal of Poverty & Social Justice, Vol. 21, Issue 1, pp. 33-45), researcher O. Lelkes explores the impact of Internet use. The...
-
Define and explain specific fuel consumption.
-
Torre Manufacturing Company obtains its raw materials from a variety of suppliers. Torres strategy is to obtain the best price by letting the suppliers know that it buys from the lowest bidder....
-
Write a method named two DimSearch that searches a 2D array row by row sequentially and prints out the row and column number of every occurrence of the value val if the array contains the requested...
-
Balance the following equations, and then classify each as a precipitation, acidbase, or gas-forming reaction. (a) Ba(OH)(aq) + HCl(aq) BaCl,(aq) + H,O(l) (b) HNO3(aq) + COCO3(s) Co(NO3)2(aq) +...
-
For each reaction, write an overall, balanced equation and the net ionic equation. (a) The reaction of aqueous lead(II) nitrate and aqueous potassium hydroxide (b) The reaction of aqueous copper(II)...
-
The data in FB represent the number of Facebook users (quarterly, in millions) worldwide between the first quarter of 2009 and the second quarter of 2018. a. Plot the series of data. b. Compute a...
-
Simplify the expression: w+-2w6+ 9 + -w
-
Simplify 343 to the form ab.
-
I am working on the topic DoorDash for marketing plan which DoorDash is considered to be an innovative service. My questions: What are ways that DoorDash currently market the service (I think both...
-
(Net present value calculation) Big Steve's, makers of swizzle sticks, is considering the purchase of a new plastic stamping machine. This investment requires an initial outlay of $90,000 and will...
-
Divide. 3x r 5 4 Submit your answer in simplified form.
-
Company S is a 70%-owned subsidiary of Company P. Company S is building a ship to be used by Company P. The ship was 40% completed in 2011 and 100% completed in 2012. The actual and budgeted profit...
-
In 1995 Miguel purchased a home for $130,000. In 2000 he sold it for $170,000 and immediately purchased another one for $180,000, which he sold in 2007 for $235,000. How much taxable capital gain, if...
-
When ethylene glycol is treated with sulfuric acid, 1, 4-dioxane is obtained. Propose a mechanism for this transformation: H,SO, Ethylene glycol 1,4-Dioxane
-
The Williamson ether synthesis cannot be used to prepare tert-butyl phenyl ether. a. Explain why this method cannot be used in this case. b. Suggest an alternative method for preparing tert-butyl...
-
Methylmagnesium bromide reacts rapidly with ethylene oxide, it reacts slowly with oxetane, and it does not react at all with tetrahydrofuran. Explain this difference in reactivity. Oxetane Ethylene...
-
Harris Fabrics computes its plantwide predetermined overhead rate annually on the basis of direct labor-hours. At the beginning of the year, it estimated that 29,000 direct labor-hours would be...
-
The Alpine House, Incorporated, is a large retailer of snow skis. The company assembled the information shown below for the quarter ended March 31: Sales Selling price per pair of skis Variable...
-
Six months after the triple homicide patrol officers get a call of a suspicious subject at 3:00 am. The complainant observes the suspect walking down the street and disappear behind a neighbors...

Study smarter with the SolutionInn App


