Oxygen atoms can combine with ozone to form oxygen: Using r H and the bond dissociation
Question:
Oxygen atoms can combine with ozone to form oxygen: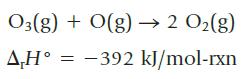
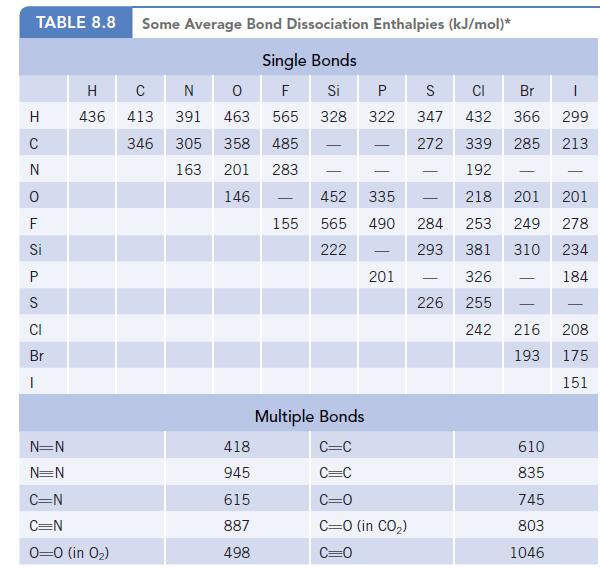
Using ΔrH° and the bond dissociation enthalpy data in Table 8.8, estimate the bond dissociation enthalpy for the oxygen–oxygen bond in ozone, O3. How does your estimate compare with the energies of an O—O single bond and an O=O double bond? Does the oxygen–oxygen bond dissociation enthalpy in ozone correlate with its bond order?
Data given in table 8.8
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





