The enthalpy change for the oxidation of naphthalene, C 10 H 8 , is measured by calorimetry.
Question:
The enthalpy change for the oxidation of naphthalene, C10H8, is measured by calorimetry.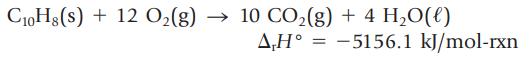
Use this value, along with the standard enthalpies of formation of CO2(g) and H2O(ℓ), to calculate the enthalpy of formation of naphthalene, in kJ/mol.
Transcribed Image Text:
C10H8(s) + 12 O₂(g) → 10 CO₂(g) + 4H₂O(l) A,H=-5156.1 kJ/mol-rxn
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
To calculate the enthalpy of formation of naphthalene C10H8 i will use the enthalpy change for t...View the full answer

Answered By

FREDRICK MUSYOKI
Professional Qualities:
Solution-oriented.
Self-motivated.
Excellent problem-solving and critical thinking skills.
Good organization, time management and prioritization.
Efficient troubleshooting abilities.
Tutoring Qualities:
I appreciate students as individuals.
I am used to tailoring resources for individual needs.
I can integrate IT into student's lessons.
I am good at explaining concepts.
I am able to help students progress.
I have a wide curriculum knowledge.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
With reference to the "Chemistry Put to Work" box on explosives, (a) Use bond enthalpies to estimate the enthalpy change for the explosion of 1.00 g of nitroglycerin. (b) Write a balanced equation...
-
The standard enthalpies of formation of ClO and ClO2 are 101 and 102 kJ/mol, respectively. Using these data and the thermodynamic data in Appendix C, calculate the overall enthalpy change for each...
-
Use Table 8.4 to estimate the enthalpy change for each of the following reactions: a. H2C == O (g) + HCl (g) H3C - O - Cl (g) b. H2O2 (g) + 2CO (g) H2 (g) + CO2 (g) (c). 3H2C == CH2 (g) C6H12 (g)...
-
What is the "presentation of self" and why is it important when we are looking at social interaction? explain
-
What is meant by the terms order qualifiers and order winners? Explain why they are important.
-
Using the demand schedule below, plot the demand curve on the graph and answer four questions about demand and elasticity: (a) Illustrate the demand curve on the following graph. (b) How much will...
-
Water flows from a two-dimensional open channel and is diverted by an inclined plate as illustrated in Fig. P5.78. When the velocity at section (1) is \(10 \mathrm{ft} / \mathrm{s}\), what horizontal...
-
The Signage Company specializes in the maintenance and repair of signs, such as billboards. On March 31, 2014, the accountant for The Signage Company prepared the following trial balances:...
-
A parallel-plate capacitor is to be constructed by using, as a dielectric, rubber with a dielectric constant of 3.20 and a dielectric strength of 15.0 MV/m. The capacitor is to have a capacitance of...
-
The enthalpy change for the oxidation of styrene, C 8 H 8 , is measured by calorimetry. Use this value, along with the standard enthalpies of formation of CO 2 (g) and H 2 O(), to calculate the...
-
An important step in the production of sulfuric acid is the oxidation of SO 2 to SO 3 . Formation of SO 3 from the air pollutant SO 2 is also a key step in the formation of acid rain. (a) Use...
-
The functions f(x) = ax 2 , where a > 0, are concave up for all x. Graph these functions for a = 1, 5, and 10, and discuss how the concavity varies with a. How does a change the appearance of the...
-
Employees at Company Q are discussing the company's decreasing market share and decreasing stock prices, but they consistently place the blame outside the company. One manager was recently reported...
-
Harmony Inc. uses a job-costing system with two direct cost categories (direct materials and direct manufacturing labor) and one manufacturing overhead cost pool. Harmony allocates manufacturing...
-
What are the key characteristics of transgressive fiction, and how does it challenge societal norms? Can you identify notable authors in this genre and discuss how their works push boundaries ?
-
(The vertices of the cube lies at x=+/-0.5333 m, y=+/-0.5333 m). Given that the electric field is +0.5. What is the flux through the cube?(Nm 2 /C) Give your answer in 4 decimal places.
-
Steven can afford car payments of $250 a month for 60 months. The bank will lend him this money at 6.2 percent interest. How much can Steven borrow?
-
What document are financial statements prepared from?
-
Classify each of the following activities as proper or prohibited under the various consumer statutes you have studied. a. Calling a hospital room to talk to a debtor who is a patient there. b....
-
Benzene(l) has a vapor pressure of 0.1269 bar at 298.15 K and an enthalpy of vaporization of 30.72 kJmol 1 . The C P,m of the vapor and liquid phases at that temperature are 82.4 and 136.0 J K 1 mol...
-
Use the values for G o f (CCl 4 , l) and G o f (CCl 4 , g) from Appendix B to calculate the vapor pressure of CCl 4 at 298.15 K.
-
Calculate the vapor pressure of water droplets of radius 1.00 10 8 m at 360. K in equilibrium with water vapor. Use the tabulated value of the density and the surface tension at 298 K from Appendix...
-
How would you integrate aspects of supervision and teambuilding into the process of leading a task group? What might that look like? What resources, including technology, could you use to ensure the...
-
The organizational level includes both the formal and the informal organization. The formal organization involves the ways authority is distributed across various organizational levels and how...
-
Effective leadership demands a deep understanding of influence tactics to achieve goals and gain support from team members. This paper examines Gary Yukl's insights on leader effectiveness, as...

Study smarter with the SolutionInn App


