The enthalpy changes of the following reactions can be measured: (a) Use these values and Hesss law
Question:
The enthalpy changes of the following reactions can be measured:
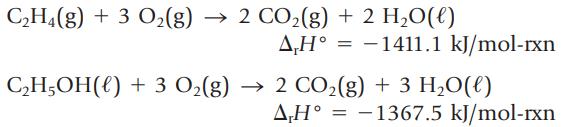
(a) Use these values and Hess’s law to determine the enthalpy change for the reaction![]()
(b) Draw an energy level diagram that shows the relationship between the energy quantities involved in this problem.
Transcribed Image Text:
CH,(g) + 3 O2(g) → 2 CO2(g) + 2 HO(0) ΔΗ° = -1411.1 kJ/mol-rxn CH,OH(0) + 3 O2(g) → 2 CO2(g) + 3 H2O(0) ΔΗ° = -1367.5 kJ/mol-rxn
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
The enthalpy changes for the following reactions can be measured: (a) Use these values and Hesss law to determine the enthalpy change for the reaction (b) Draw an energy level diagram that shows the...
-
The standard molar enthalpy of formation of diborane, B 2 H 6 (g), cannot be determined directly because the compound cannot be prepared by the reaction of boron and hydrogen. It can be calculated...
-
An important step in the production of sulfuric acid is the oxidation of SO 2 to SO 3 . Formation of SO 3 from the air pollutant SO 2 is also a key step in the formation of acid rain. (a) Use...
-
A 40-cm-long, 800-W electric resistance heating element with diameter 0.5 cm and surface temperature 120C is immersed in 75 kg of water initially at 20C. Determine how long it will take for this...
-
Your assignment is to analyze the data collected by Dr. Bay. In particular, you should 1. Describe how Dr. Bay can use the data. 2. Suggest ways in which the administrative assistants jobs can be...
-
A cap on a floating-rate euro loan: (a) Protects the borrower against high short-term interest rates. (b) Protects the lender against high short-term interest rates on the funding side. (c) Is...
-
A nozzle is designed to accelerate the fluid from \(V_{1}\) to \(V_{2}\) in a linear fashion. That is, \(V=a x+b\), where \(a\) and \(b\) are constants. If the flow is constant with \(V_{1}=10...
-
An amusement park, whose customer set is made up of two markets, adults and children, has developed demand schedules as follows: The marginal operating cost of each unit of quantity is $5. (Hint:...
-
1. Optimize the following Boolean function F together with the don't care conditions d. F(x, y, z, t) IIM(1, 5, 7, 8, 9, 11, 13) = F(x,y,z,t)=Em(0, 4, 6, 12) (a) (4 points) Draw four-variable...
-
An ice calorimeter can be used to determine the specific heat capacity of a metal. A piece of hot metal is dropped onto a weighed quantity of ice. The energy transferred from the metal to the ice can...
-
A 0.692-g sample of glucose, C 6 H 12 O 6 , was burned in a constant-volume calorimeter. The temperature rose from 21.70C to 25.22C. The calorimeter contained 575 g of water, and the bomb had a heat...
-
In Exercises 4752, graph functions f and g in the same rectangular coordinate system. Graph and give equations of all asymptotes. If applicable, use a graphing utility to confirm your hand-drawn...
-
A political and economic system in which the government uses its authority to promote social and economic equality, providing everyone with basic services and equal opportunities and requiring...
-
In the digital age of marketing, special care must be taken to make sure that programmatic ads appearing on websites align with a company's strategy, culture and ethics. For example, in 2017,...
-
Hosaia, LLC is a manufacturer of cabinets. Hosaia introduces all materials at the beginning of production. Throughout the manufacturing process, conversion cost is incurred evenly. The quality...
-
A restaurant is catering an off site event. Sales will be $ 2 , 7 5 0 . They will budget 3 2 . 5 % of those sales for labor cost. How much money will budgeted for labor cost?
-
You pay $10 for access to a dessert bar that serves unlimited slices of cheesecake. Is this price discrimination in terms of the slices of cheesecake?
-
Of the three approaches to calculate sales required to achieve target profit, which one(s) calculate the required sales in units and which one(s) calculate the required sales in dollars?
-
Classify each of the following as direct costs or indirect costs of operating the Pediatrics ward for children at the Cleveland Clinic: a. Wi-Fi covering the entire hospital campus b. Net cost of...
-
For each pair of compounds below, identify the one that would be expected to have more ionic character. Explain your choice. a) NaBr or HBr b) BrCl or FCl
-
Is the expression only valid for an ideal gas if V is constant? T, AUr = [ CydT = n[ Cy,maT
-
Draw a Lewis dot structure for each of the following compounds: a. CH 3 CH 2 OH b. CH 3 CN
-
Xplornet Ltd. is an internet service provider. The company had 14,000,000 subscribers in 2021, and employed 18 customer service representatives. During 2021 each customer service representative...
-
An accounting department plays an enormous role within the medical office. As the backbone of the organization, the accounting department allows the organization to operate at its fullest potential....
-
Two cylinders are similar. The volume of one is 27 cm, and the volume of the other is 1331 cm. Find the scale factor between them. Enter the smaller number first. scale factor = [?] : [ ] Enter

Study smarter with the SolutionInn App


