The enthalpy changes for the following reactions can be measured: (a) Use these values and Hesss law
Question:
The enthalpy changes for the following reactions can be measured: 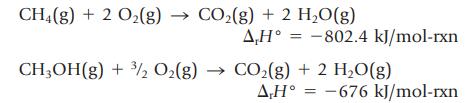
(a) Use these values and Hess’s law to determine the enthalpy change for the reaction![]()
(b) Draw an energy level diagram that shows the relationship between the energy quantities involved in this problem.
Transcribed Image Text:
CH4(g) + 2 O₂(g) → CO₂(g) + 2 H₂O(g) A,H° = -802.4 kJ/mol-rxn CH₂OH(g) + 3/2 O₂(g) → CO₂(g) + 2 H₂O(g) AH° -676 kJ/mol-rxn
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Energy a AH 126 kJmolrxn b CH4g 12 O...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
The enthalpy changes of the following reactions can be measured: (a) Use these values and Hesss law to determine the enthalpy change for the reaction (b) Draw an energy level diagram that shows the...
-
The standard molar enthalpy of formation of diborane, B 2 H 6 (g), cannot be determined directly because the compound cannot be prepared by the reaction of boron and hydrogen. It can be calculated...
-
The standard enthalpy of formation of solid barium oxide, BaO, is 553.5 kJ/mol, and the standard enthalpy of formation of barium peroxide, BaO 2 , is 634.3 kJ/mol. (a) Calculate the standard enthalpy...
-
Please help me calculate the current assets and current liabilities. Cash and cash equivalents Deposits Marketable securities Inventory Property & equipment, net Loan to shareholders Notes receivable...
-
Your company has received an order for 20 units of a product. The labor cost to produce the item is $9.50 per hour. The setup cost for the item is $60 and material costs are $25 per unit. The item is...
-
Sophie is a single taxpayer. For the first payroll period in October 2013, she is paid wages of $3,250 monthly. Sophie claims three allowances on her Form W-4. a. Use the percentage method to...
-
With reference to Exercise 4.5, find an expression for the distribution function \(F(x)\) of the random variable. Data From Exercise 4.5 k 4.5 Given that f(x): = is a probability distribution for 2x...
-
Liane Hansen has prepared the following list of statements about bonds. 1. Bonds are a form of interest-bearing notes payable. 2. When seeking long-term financing, an advantage of issuing bonds over...
-
Bramble Corp, applies overhead on the basis of machine hours. Given the following data, what is the amount of overhead applied and the amount by which it is under- or overapplied for the period?...
-
A 0.692-g sample of glucose, C 6 H 12 O 6 , was burned in a constant-volume calorimeter. The temperature rose from 21.70C to 25.22C. The calorimeter contained 575 g of water, and the bomb had a heat...
-
Suppose you burned 1.500 g of benzoic acid, C 6 H 5 CO 2 H, in a constant-volume calorimeter and found that the temperature increased from 22.50C to 31.69C. The calorimeter contained 775 g of water,...
-
Use the following equation to derive a demand schedule and a demand curve. What types of products might exhibit this type of nonlinear demand curve? Explain. Q = 100P-0.3
-
What is community development? Why is community development and community participation important? What is the role of Community Development workers in the process?
-
speculator may write a put option on stock with an exercise price of $15 and earn a $3 premium only if he thought a-the stock price would rise above $18 or fall below $ 12. b-the stock price would...
-
Franklin Corporation just paid taxes of $152,000 on taxable income of $512,000. The marginal tax rate is 35% for the company. What is the average tax rate for the Franklin Corporation?
-
what are the four domains of human service and the concept of social justice. And define the role of Professional values.
-
FallSafe Company expects exponential growth in the very near future. How do you think it can most effectively plan for expansion, and what tools of cost-volume-profit Analysis can/should it use?
-
According to ars technica, HP is the leading company in the United States in PC sales with about 27% of the market share. Suppose a business researcher randomly selects 130 recent purchasers of PCs...
-
Separate variables and use partial fractions to solve the initial value problems in Problems 18. Use either the exact solution or a computer-generated slope field to sketch the graphs of several...
-
The molar heat capacity C P,m of SO 2 (g) is described by the following equation over the range 300 K < T < 1700 K: In this equation, T is the absolute temperature in kelvin. The ratios T/K ensure...
-
Use the relation (U/V ) T = T(P/T) V P and the cyclic rule to obtain an expression for the internal pressure, (0U/0V )T , in terms of P, , T, and .
-
A mass of 34.05 g of H 2 O(s) at 273 K is dropped into 185 g of H 2 O(l) at 310.K in an insulated container at 1 bar of pressure. Calculate the temperature of the system once equilibrium has been...
-
Make up an advertisement about a water bottle that uses at least two of the three methods of persuasion (pathos, logos, ethos) and uses demographics to appeal to a specified target audience. Then...
-
Audit the channel functions by using the efficiency template which involves evaluating the effectiveness of a company's marketing and sales channels using a set of criteria that focus on efficiency....
-
As a health care manager, it is important to understand and explore methods to advocate for change within the industry through policy analysis. It is also important to be able to navigate the...

Study smarter with the SolutionInn App


