Water gas, a mixture of carbon monoxide and hydrogen, is produced by treating carbon (in the form
Question:
Water gas, a mixture of carbon monoxide and hydrogen, is produced by treating carbon (in the form of coke or coal) with steam at high temperatures. (See Study Question 83.![]()
Not all of the carbon available is converted to water gas since some is burned to provide the heat for the endothermic reaction of carbon and water. What mass of carbon must be burned (to CO2 gas) to provide the energy to convert 1.00 kg of carbon to water gas?
Data given in Study Question 83
When heated to a high temperature, coke (mainly carbon, obtained by heating coal in the absence of air) and steam produce a mixture called water gas, which can be used as a fuel or as a starting place for other reactions. The equation for the production of water gas is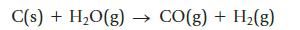
Step by Step Answer:

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel





