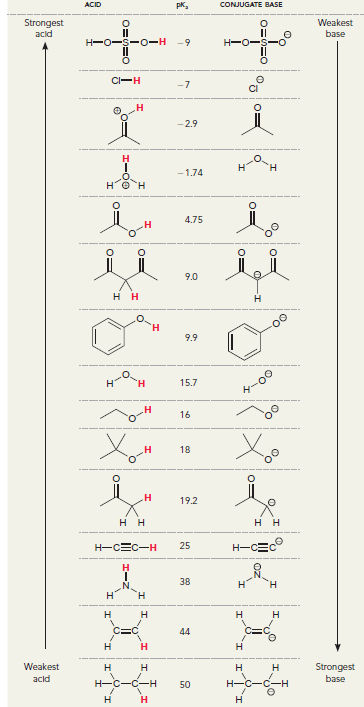
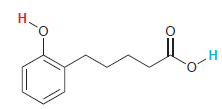
In the following compound two protons are clearly identified. Determine which of the two is more acidic.
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





