You have an irregularly shaped piece of an unknown metal. To identify it, you determine its density
Question:
You have an irregularly shaped piece of an unknown metal. To identify it, you determine its density and then compare this value with known values that you look up in the chemistry library. The mass of the metal is 74.122 g. Because of the irregular shape, you measure the volume by submerging the metal in water in a graduated cylinder. When you do this, the water level in the cylinder rises from 28.2 mL to 36.7 mL.
(a) What is the density of the metal? (Use the correct number of significant figures in your answer.)
(b) The unknown is one of the seven metals listed below. Is it possible to identify the metal based on the density you have calculated? Explain.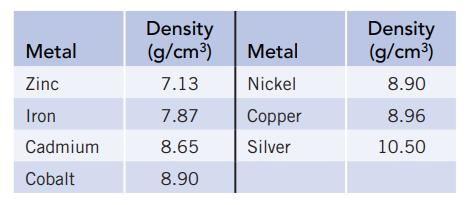
Step by Step Answer:

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel





