Calculate the standard free energy change for the following reactions using the standard cell potentials for the
Question:
Calculate the standard free energy change for the following reactions using the standard cell potentials for the half-reactions that are involved.
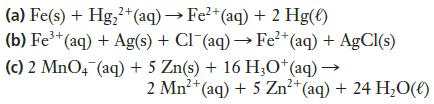
Transcribed Image Text:
2+ (a) Fe(s) + Hg₂+ (aq) → Fe²+ (aq) + 2 Hg(e) (b) Fe³+ (aq) + Ag(s) + Cl (aq) → Fe²+ (aq) + AgCl(s) (c) 2 MnO4 (aq) + 5 Zn(s) + 16 H3O+ (aq) → 2+ 2 Mn²+ (aq) + 5 Zn²+ (aq) + 24 H₂O(l)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (7 reviews)
a 237 ...View the full answer

Answered By

FREDRICK MUSYOKI
Professional Qualities:
Solution-oriented.
Self-motivated.
Excellent problem-solving and critical thinking skills.
Good organization, time management and prioritization.
Efficient troubleshooting abilities.
Tutoring Qualities:
I appreciate students as individuals.
I am used to tailoring resources for individual needs.
I can integrate IT into student's lessons.
I am good at explaining concepts.
I am able to help students progress.
I have a wide curriculum knowledge.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
In Example Problem 10.1, we considered an addition reaction involving two ethylene molecules and found that the entropy change was negative. We suggested at the time that this reaction would still be...
-
Calculate the standard free energy change for the dissociation of HEPES.
-
The standard free energy change for the hydrolysis of ATP was given in Problem 91. In a particular cell, the concentrations of ATP, ADP, and P i are 0.0031 M, 0.0014 M, and 0.0048 M, respectively....
-
1 - Describe the purpose of the bubble sort algorithm 2 - The pseudocode below shows the working of the main condition in a bubble sort algorithm, but one part of the code is missinIF items [ i ] >...
-
When might persuasion be necessary in messages flowing upward?
-
Hoboken Industries currently manufactures 30.000 units of part MR24 each month for use in production of several of its products. The facilities now used to produce part MR24 have a fixed monthly cost...
-
Copy your worksheet from Question 6 into another worksheet. Change the increase from 10% to 18%. Protect the worksheet, so that changes cannot be made. Question 6 Open a new spreadsheet. Type...
-
Marston Marble Corporation is considering a merger with the Conroy Concrete Company. Conroy is a publicly traded company, and its current beta is 1.30. Conroy has been barely profitable, so it has...
-
On a private flight with capacity for 150 people, $800,000 per person is charged, plus $25,000 for each unsold seat on the plane. How many people must travel on the plane and what will be the price...
-
Suppose that you cannot find a table of standard reduction potentials. You remember that the standard reduction potential of Cu 2+ + 2 e Cu(s) is 0.337 V. Given that Gf(Cu 2+ ) = 65.49 kJ mol 1 and...
-
In May 2000, a concrete pedestrian walkway collapsed in North Carolina, injuring more than 100 people. Investigation revealed that CaCl 2 had been mixed into the grout that filled the holes around...
-
Spotify is a music streaming platform that gives access to songs from artists all over the world. On February 28, 2018, Spotify filed for an initial public offering (IPO) on the New York Stock...
-
4. Michelle received $6500 as a personal loan to be paid off in 3 years, but she has already made two payments: $1500 in a year, and $2250 in 2 years. How much is left to be paid at the end of her...
-
Suppose you were to deposit $928.00 into a savings account that earns 1.8% interest compounded continuously. Use the continuously compounding interest formula A = Pert to answer the following. a) How...
-
Question: 1 Assume that the spread is GBP 1 = 1.5500-1.6500 USD. Consider the situations of three UK based companies: (a) K Ltd imports goods from Texas to the value of USD100,000. Payment is cash on...
-
A taxpayer buys several lots of Bitcoin for a combined total of $12,500 during TY2023. They convert half of the Bitcoin to Ethereum when the original lot of Bitcoin is worth $20,000. Later, Ethereum...
-
A founder holds 2 million common shares of GeoTech Company. In 2018, Sunset Ventures bought 2 million preferred shares for $2 million. The preferred shares are convertible into one common share with...
-
For the table given, repeat Prob. 117 for the following O rings, given the AS 568A standard number. Repeat Prob. 117, A circular cross section O ring has the dimensions shown in the figure. In...
-
Explain the differences and similarities between fringe benefits and salary as forms of compensation.
-
The solid shaft has a linear taper from r A at one end to r B at the other. Derive an equation that gives the maximum shear stress in the shaft at a location x along the shafts axis.
-
The assembly shown consists of two A992 steel bolts AB and EF and an 6061-T6 aluminum rod CD. When the temperature is at 30° C, the gap between the rod and rigid member AE is 0.1 mm. Determine...
-
The assembly consists of two A992 steel bolts AB and EF and an 6061-T6 aluminum rod CD. When the temperature is at 30° C, the gap between the rod and rigid member AE is 0.1 mm. Determine the...
-
Some cost items are more of a reflection of the strategy of the company and are not directly linked to revenue in a given period. For example, some companies spend large amounts advertising their...
-
Why does the GASB require governments to include both original and revised budget data in a budgetary comparison statement (or schedule)?
-
If the labour variances are as follows would you suggest the proposal: Price variance 1,800 F, Efficiency variance, 1,200 U. The materials were purchased from a new supplier who is anxious to enter...

Study smarter with the SolutionInn App


