Determine the shape of each of the following species. (a) P O 4 3 , (b) PCl
Question:
Determine the shape of each of the following species.
(a) P O 4 3–,
(b) PCl5
Strategy Draw the Lewis structures. Each of these molecules has only single bonds around its central atom. So we can count the number of bonding pairs around the central atom and assign the geometry by consulting Table 7.3 as needed.
Table 7.3
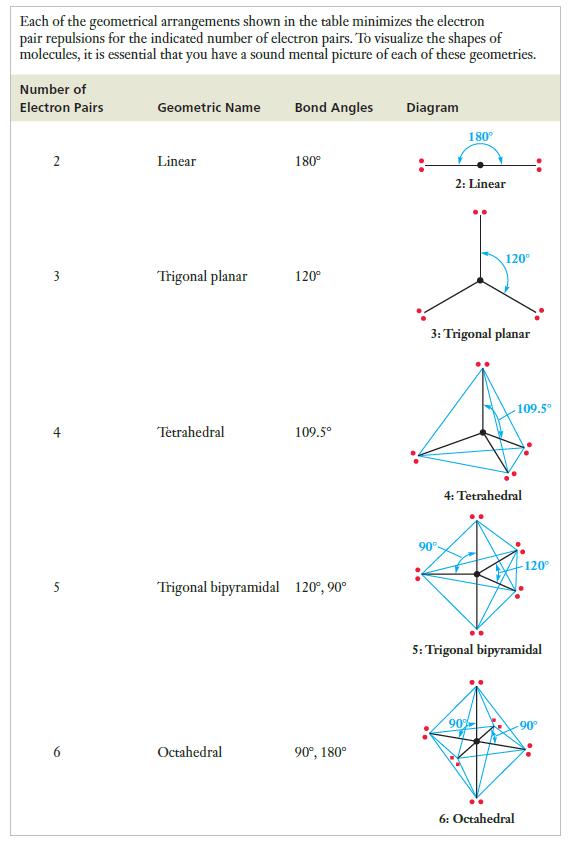
Transcribed Image Text:
Each of the geometrical arrangements shown in the table minimizes the electron pair repulsions for the indicated number of electron pairs. To visualize the shapes of molecules, it is essential that you have a sound mental picture of each of these geometries. Number of Electron Pairs 2 3 5 6 Geometric Name Linear Trigonal planar Tetrahedral Bond Angles Octahedral 180° 120° 109.5⁰ Trigonal bipyramidal 120°, 90° 90⁰, 180° Diagram 180° 90° 2: Linear 3: Trigonal planar 120⁰ 4: Tetrahedral 90% 109.5° 5: Trigonal bipyramidal 6: Octahedral -120° -90°
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a The Lewis structure of P O 4 3 which we drew in Example Problem 75 is The central phosphorus atom has a complete octet of electrons contributed by f...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Use VSEPR theory to determine the shape of the NOF molecule. Strategy Once again, we start by drawing the Lewis structure. Then count the number of regions of electron density around the central...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The histograms show the number of pairs of shoes reported for 300 males and for 300 females. Descriptive statistics are also shown. a. Describe the shape of each histogram. b. Because of the shapes,...
-
(a) Find the first order fraction transformation where z 1 =,z 2 =0, z 3 =1 is thought to be w 1 =1, w 2 =i, w 3 =-1 each. (b) Find the anchor points of w=(z-1)/(z+1)
-
How would the analysis be different if Hagers intended to recapitalize Lyons with 40% debt costing 10% at the end of four years? This amounts to $221.6 million in debt as of the end of 2013. The...
-
Alices Alterations has eight jobs to be completed and only one sewing machine (and sewing machine operator). Given the processing times and due dates as shown here, prioritize the jobs by SPT, DDATE,...
-
True or False: If \(\operatorname{IRR}(\mathrm{A})>\operatorname{IRR}(\mathrm{B})\), then \(\operatorname{ERR}(\mathrm{A})>\operatorname{ERR}(\mathrm{B})\).
-
Karls Copiers sells and repairs photocopy machines. The manager needs weekly forecasts of service calls so that he can schedule service personnel. Use the actual demand in the first period for the...
-
. Do policies exist within the Linux workstation or server environment? If so, what are they? . Are there ways that Windows policies can be leveraged to enhance corporate or security policy?
-
Why is the Na 2+ ion not found in nature?
-
Use the concept of polarity of water and the basic composition of the body to explain why the polarity of biomaterials is important.
-
Convert the following radian measures to degrees. 5
-
A new minister responsible for retirement income strategy in the Finland to has commissioned a report from you. The report is to provide background information to assist the minister in improving...
-
ESSAY PRACTICE FOR EXAM PAYOFF MATRIX QUESTIONS B\A A A MANGOES PEACHES B MANGOES 5/14 10/10 B PEACHES 12/12 14/5 (a) Explain what As best response is if B Plants MANGOES AND what would be A's best...
-
Suppose f is the function defined by: f(x)=x^(2)+7x+12 (a) Find the vertex of f(x) (b) Find the x-intercept s (also known as the zeros ) of f(x) if they exist
-
The strength of the local and national economies is often measured by the unemployment rate. The total workforce includes the number of people who are employed and the number of people actively...
-
Save your program as Project1.java (...this implies the name of your Class. Don't forget!), ZIP it into a zip file names Project1.zip, and submit Project1.zip to Canvas in the Project 1 link by the...
-
Each of the four independent situations below describes a direct financing lease in which annual lease payments of $10,000 are payable at the beginning of each year. Each is a capital lease for the...
-
In Problems 1522, find the principal needed now to get each amount; that is, find the present value. To get $750 after 2 years at 2.5% compounded quarterly.
-
Hydroxymethylene has never actually been observed, although it is believed to be an intermediate both in the photo-fragmentation of formaldehyde to hydrogen and carbon monoxide, and in the...
-
The three vibrational frequencies in H 2 O (1595, 3657, and 3756 cm 1 ) are all much larger than the corresponding frequencies in D 2 O (1178, 2671, and 2788 cm 1 ). This follows from the fact that...
-
DielsAlder reactions commonly involve electron-rich dienes and electron-deficient dienophiles: The rate of these reactions generally increases with the Ï -donor ability of the diene substituent,...
-
Suppose Cute Camel Woodcraft Company is evaluating a proposed capital budgeting project (project Alpha) that will require an initial investment of $400,000. The project is expected to generate the...
-
This case study builds on knowledge learnt from the first half of the unit and previous assessments and asks you to showcase your analytical skills in strategic evaluation, critical thinking,...
-
The controller of Fortnight Company has requested a quick estimate of the manufacturing supplies needed for the Cleveland Plant for the month of July, when production is expected to be 520,000 units...

Study smarter with the SolutionInn App


