Figure 7.2 depicts the interactions of an ion with its first nearest neighbors, second nearest neighbors, and
Question:
Figure 7.2 depicts the interactions of an ion with its first nearest neighbors, second nearest neighbors, and third nearest neighbors in a lattice.
Figure 7.2
(a) Would the interactions with the fourth nearest neighbors be attractive or repulsive?
(b) Based on Coulomb’s law, how would the relative sizes of the terms compare if the potential energy were expressed as V = V1st + V2nd + V3rd + V4th?
Transcribed Image Text:
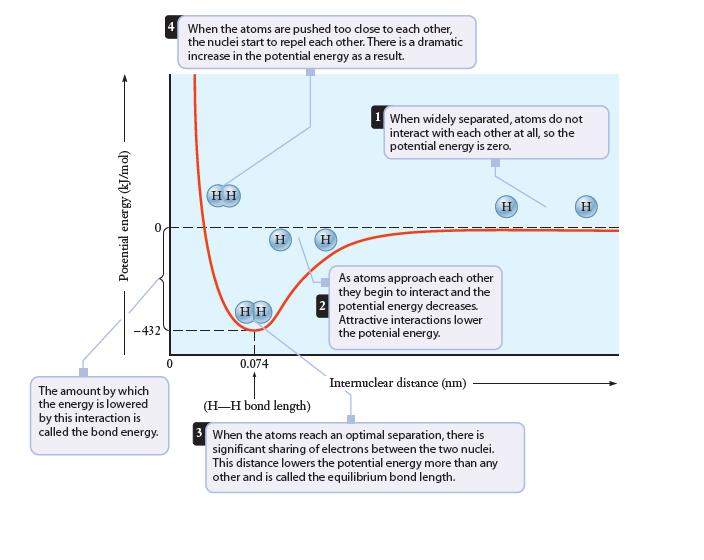
Potential energy (kJ/mol) -432 The amount by which the energy is lowered by this interaction is called the bond energy. 4 When the atoms are pushed too close to each other, the nuclei start to repel each other. There is a dramatic increase in the potential energy as a result. 0 HH HH 0.074 H H 1 When widely separated, atoms do not interact with each other at all, so the potential energy is zero. As atoms approach each other they begin to interact and the 2 potential energy decreases. Attractive interactions lower the potenial energy. Internuclear distance (nm) (H-H bond length) 3 When the atoms reach an optimal separation, there is significant sharing of electrons between the two nuclei. This distance lowers the potential energy more than any other and is called the equilibrium bond length. H H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a Repulsive b According to Equation 72 the C...View the full answer

Answered By

Cyrus Sandoval
I a web and systems developer with a vast array of knowledge in many different front end and back end languages, responsive frameworks, databases, and best code practices. My objective is simply to be the best web developer that i can be and to contribute to the technology industry all that i know and i can do. My skills include:
- Front end languages: css, HTML, Javascript, XML
- Frameworks: Angular, Jquery, Bootstrap, Jasmine, Mocha
- Back End Languages: Java, Javascript, PHP,kotlin
- Databases: MySQL, PostegreSQL, Mongo, Cassandra
- Tools: Atom, Aptana, Eclipse, Android Studio, Notepad++, Netbeans.
Having a degree in Computer Science enabled me to deeply learn most of the things regarding programming, and i believe that my understanding of problem solving and complex algorithms are also skills that have and will continue to contribute to my overall success as a developer.
I’ve worked on countless freelance projects and have been involved with a handful of notable startups. Also while freelancing I was involved in doing other IT tasks requiring the use of computers from working with data, content creation and transcription.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
1. How strong are the competitive forces confronting J. Crew in the market for specialty retail? Do a [Michael Porter] five-forces analysis to support your answer. (see chapter 3 in the textfor...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Automobiles air bags are filled via the decomposition of sodium azide, according to the following equation: 2 NaN3 (s) 2 Na (s) +3 N2 (g) Calculate the work (in L atm) when 2.25 g of sodium azide...
-
What are holding companies? What are their advantages and disadvantages? Hagers Home Repair Company, a regional hardware chain that specializes in do-it-yourself materials and equipment rentals, is...
-
Demand for the popular water toy Sudsy Soaker has far exceeded expectations. In order to increase the availability of different models of the toy, the manufacturer has decided to begin producing its...
-
Quilts R Us (QRU) is considering investing in a new patterning attachment with the cash flow profile shown in the table below. QRU's MARR is 13.5 percent/year. a. What is the internal rate of return...
-
Gravois, Inc., incurred the following costs during June: Selling expenses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . $158,375 Direct labor . . . . . . . . . . . . . . . . ....
-
Two blocks are fastened to the ceiling of an elevator as in Figure. The elevator accelerates upward at 2.00 m/s. Find the tension in each rope. T 10.0 kg T 10.0 kg
-
Arrange the following sets of anions in order of increasing ionic radii. (a) Cl , P 3 , S 2 , (b) S 2 , O 2 , Se 2 , (c) Br , N 3 , S 2 , (d) Br , Cl , I
-
Select the smaller member of each of the following pairs. (a) N and N 3 , (b) Ba and Ba 2+ , (c) Se and Se 2 , (d) Co 2+ and Co 3+
-
It is a commonly held belief that SUVs are safer than cars. If an SUV and car are in a collision, does the SUV sustain less damage (as suggested by the cost of repair)? The Insurance Institute for...
-
Describe why feasibility studies are necessary. How is a SWOT analysis used to develop a marketing plan Explain why it is necessary to segment. What are the elements that make up segmentation How...
-
Explain how Base Erosion Profit Shifting (BEPS) initiative is changing the dynamics of Double Tax Agreements (DTA) and Transfer Pricing regimes around the world and how these will impact on Fiji's...
-
Why will profit-maximizing managers never use a dominated strategy?
-
How can insurance be used to transfer risk? Provide a simple example of a risk that could be transfered by writing an insurance policy.
-
What is a risk register and why is it used? What is project risk control? Why is it important? When it is important to create a risk assessment chart? What is contained in a risk mitigation matrix?...
-
You are the new controller for Moonlight Bay Resorts. The company CFO has asked you to determine the company's interest expense for the year ended December 31, 2011. Your accounting group provided...
-
A routine activity such as pumping gasoline can be related to many of the concepts studied in this text. Suppose that premium unleaded costs $3.75 per gal. Work Exercises in order. Use the...
-
In the polyproline spectroscopic ruler experiment shown in Figure 25.19, the FRET pair employed is comprised of the fluorescent dyes Alexa Fluor 488 (excited-state lifetime of 4.1 ns) and Alexa Fluor...
-
For many years, a controversy raged concerning the structures of so-called electron-deficient molecules; that is, molecules with insufficient electrons to make normal two-atom, two-electron bonds....
-
One of the most powerful attractions of quantum chemical calculations over experiments is their ability to deal with any molecular system, stable or unstable, real or imaginary. Take as an example...
-
Your friend Alex comes to you with his plan to start a business. He brings you the projected financial statements for the first two years, which you carefully analyze to compute the Free Cash Flow...
-
Product Profitability Analysis Galaxy Sports Inc. manufactures and sells two styles of All Terrain Vehicles (ATVS), the Conquistador and Hurricane, from a single manufacturing facility. The...
-
what extent does socialization influence political attitudes, ideologies, and participation within democratic societies?

Study smarter with the SolutionInn App


