HBr is oxidized in the following reaction: (a) Show that this mechanism can account for the correct
Question:
HBr is oxidized in the following reaction:
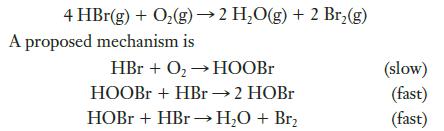
(a) Show that this mechanism can account for the correct stoichiometry.
(b) Identify all intermediates in this mechanism.
(c) What is the molecularity of each elementary step?
(d) Write the rate expression for each elementary step.
(e) Identify the rate-determining step.
Transcribed Image Text:
4 HBr(g) + O₂(g) → 2 H₂O(g) + 2 Br₂(g) A proposed mechanism is HBr + O₂ → HOOBr HOOBr + HBr → 2 HOBr HOBr + HBr → H₂O + Br₂ (slow) (fast) (fast)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a To show that the mechanism accounts for the correct stoichiometry lets sum up the elementary steps ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Show that the following mechanism can account for the rate law of the reaction in Problem 22.11: What further tests could you apply to verify this mechanism? HCl + HCl K, HCI + CH,CH=CH, complex...
-
A small drop of liquid is squeezed between two flat plates to form a thin disc of height H and diameter D. The contact angle of the liquid interface at the solid surfaces is zero, so that the...
-
A pipe-laying crew consists of two hydraulic excavators, a front-end loader, a trench box, a gravel box, a foreperson, a pipe layer, two equipment operators, and a laborer. The cost of a hydraulic...
-
Match each of the following energy band structures with the type of material it represents. Show Work A B C Empty conduction band Band gap Filled valence band Empty conduction band Band gap Filled...
-
The company had 50,000 shares of common stock outstanding throughout the year. In addition, as of January 1, the company had issued 100 convertible bonds ($1,000 face value, 10%). The company has no...
-
Hewitt Companys output for the current period yields a $30,000 favorable overhead volume variance and a $50,400 unfavorable overhead controllable variance. Standard overhead charged to production for...
-
Develop the appropriate primary research question to be associated with this design. Develop a hypothetical research scenario that would necessitate the use of the Action Research Approach and a...
-
Aikman (beginning capital, $60,000) and Rory (beginning capital $90,000) are partners. During 2012, the partnership earned net income of $70,000, and Aikman made drawings of $18,000 while Rory made...
-
When controlling for many factors that determine earnings differentials (age, occupation, education, etc.), there is still an 'unexplained' difference of 11% in earnings between Black and white...
-
In the process by which CFCs contribute to the depletion of stratospheric ozone, is light a catalyst? Why or why not?
-
The following mechanism is proposed for a reaction: (a) Write the overall equation for the reaction. (b) What is the rate-determining step? (c) What is the intermediate in this reaction? (d) What is...
-
Storrs Cycles has just started selling the new Cyclone mountain bike, with monthly sales as shown in the table. First, co- owner Bob Day wants to forecast by exponential smoothing by initially...
-
"What are the implications of empowerment for talent management and human resource development? Furthermore, what strategies can be implemented to empower employees through training, mentoring, and...
-
Discuss the challenges and opportunities associated with cross-cultural delegation, considering factors such as cultural values, communication styles, and power distance dimensions in shaping...
-
7. You want to buy a house and will need to borrow $280,000. The interest rate on your loan is 6.19 percent compounded monthly and the loan is for 20 years. What are your monthly mortgage payments?
-
Claire Gerber wants to buy 400 shares of Google, which is selling in the market for $534.14 a share. Rather than liquidate all her savings, she decides to borrow through her broker at 5 percent a...
-
An employee is arriving late to a meeting and she gets to her computer so fast that her boss s 2 8 2 . 3 Hz tenor voice is shifted 2 . 3 Hz higher. How fast was she moving?
-
Below is the net income of Jonesey Laboratories computed under the three inventory methods. Instructions (Ignore tax considerations.) (a) Assume that in 2015 Jonesey decided to change from the...
-
The Adjusted Trial Balance columns of a 10-column work sheet for Webber Co. follow. Complete the work sheet by extending the account balances into the appropriate financial statement columns and by...
-
Consider the following mechanism, which results in the formation of product P: If only the species A is present at t = 0, what is the expression for the concentration of P as a function of time? You...
-
Using the preequilibrium approximation, derive the predicted rate law expression for the following mechanism: A+B- P
-
For the reaction I (aq) + OCl (aq) OI (aq) + Cl (aq) occurring in aqueous solution, the following mechanism has been proposed: a. Derive the rate law expression for this reaction based on this...
-
Monty Corporation has the following four items in its ending inventory: Item Cost Estimated Selling Price Estimated Disposal Costs Neutrinos $ 1,910 $2,190 $115 Ocillinos 5,060 4,960 107 Electrons...
-
This information is for Novak Company for the year ended December 31, 2022. Cash received from revenues from customers $648,500 Cash received for issuance of common stock 290,000 Cash paid for new...
-
On January 1 of Year 1, Baker Corp. purchased $40,000 of Chocolate Inc. bonds. These bonds pay 5% interest annually on December 31 and mature in 10 years on December 31. The investment is classified...

Study smarter with the SolutionInn App


