Hydrogen sulfide, H 2 S, is present in many oil products, including natural gas from many sources.
Question:
Hydrogen sulfide, H2S, is present in many oil products, including natural gas from many sources. The removal of this sulfur-containing molecule involves several steps, including the following reaction:
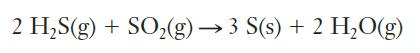
Studying this reaction in the laboratory requires extra safety precautions because of the toxicity of some of the reacting gases. If 19.6 L of H2S at 722 torr and 17°C reacts with excess SO2, what mass of solid sulfur could be produced?
Strategy We are asked to find the mass of a reaction product, so this is a reaction stoichiometry problem. Because we are told that excess SO2 is present, we know that H2S must be the limiting reactant. So we should begin by finding the number of moles of H2S that react. The only new wrinkle here is that instead of being given the mass of H2S, we are given P, V, and T. If we assume that the gas behaves ideally, we can easily use the gas law to get the number of moles of H2S that react. Once we know that we can use the balanced equation for the reaction to identify the correct mole ratio and solve.
Step by Step Answer:

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme





