One way of determining blood alcohol levels is by performing a titration on a sample of blood.
Question:
One way of determining blood alcohol levels is by performing a titration on a sample of blood. In this process, the alcohol from the blood is oxidized by dichromate ions (Cr2O7 2-) according to the following net ionic equation:
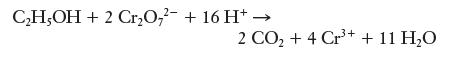
A 10.00-g sample of blood was drawn from a patient, and 13.77 mL of 0.02538 M K2Cr2O7 was required to titrate the alcohol. What was the patient’s blood alcohol level? (See the previous problem for definition of blood alcohol level. K2Cr2O7 is a strong electrolyte, so it dissociates completely in solution.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





