The combustion of acetylene was used in welders torches for many years because it produces a very
Question:
The combustion of acetylene was used in welder’s torches for many years because it produces a very hot flame:
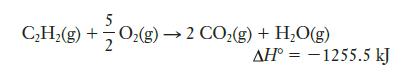
(a) Use data in Appendix E to calculate ΔS° for this reaction.
(b) Calculate ΔG° and show that the reaction is spontaneous at 25°C.
(c) Is there any temperature range in which this reaction is not spontaneous?
(d) Do you think you could use Equation 10.4 to calculate such a temperature range reliably? Explain your answer.
Equation 10.4
![]()
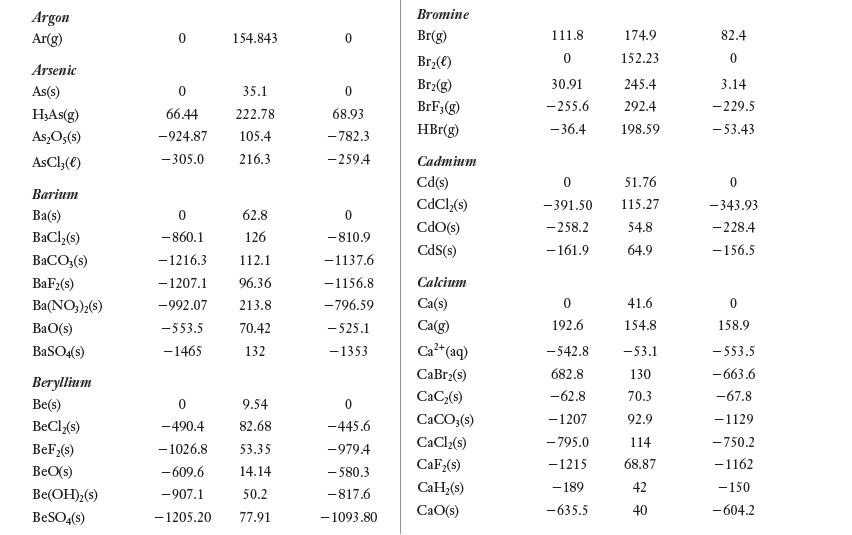
Appendix E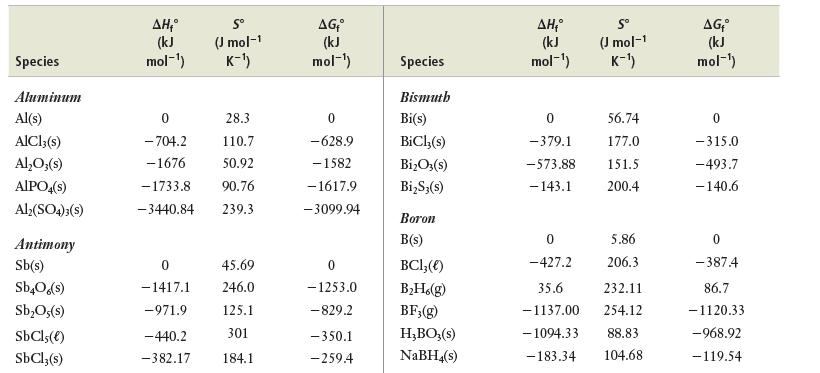
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





