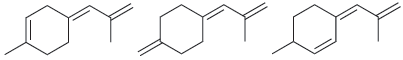
Question: Identify the most stable compound:
Step by Step Solution
3.37 Rating (175 Votes )
There are 3 Steps involved in it
In the com... View full answer

Get step-by-step solutions from verified subject matter experts

3.37 Rating (175 Votes )
There are 3 Steps involved in it
In the com... View full answer

Get step-by-step solutions from verified subject matter experts