The reaction, 3 H 2 (g) + N 2 (g) 2 NH 3 (g), has the
Question:
The reaction, 3 H2(g) + N2(g) ⇄ 2 NH3(g), has the following equilibrium constants at the temperatures given:
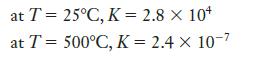
(a) At which temperature are reactants favored?
(b) At which temperature are products favored?
(c) What can you say about the reaction if the equilibrium constant is 1.2 at 127°C?
Transcribed Image Text:
at T = 25°C, K = 2.8 × 10+ at T = 500°C, K = 2.4 x 10-7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a At which temperature are reactants favored At a given temperature if the equilibrium constant K is ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
You place the substance A(g) in a container. Consider the following reaction under standard conditions to produce the substance B(g): For this reaction as written, the equilibrium constant is a very...
-
How do you draw a network diagram in a network drawing program?
-
Given the following equilibrium constants at 427oC, Na2O(s) 2Na(l) + 12 O2(g) K1 = 2 10-25 NaO(g) Na(l) + l2 O2(g) K2 = 2 10-5 Na2O2(s) 2Na(l) + O2(g) K3 = 5 10-29 NaO2(s) Na(l) + O2(g) K4 = 3...
-
How to find the expected utility of profit under each alternative crop Table 1. Annual Returns to Cropping Alternatives ($ profit / acre) Outcome Worst Bad OK Good Great Probability 0.1 0.2 0.4 0.2...
-
Will Samsung likely achieve its goals in markets where it does not dominate, such as smart phones? Why or why not? In the world of consumer electronics, copycat brands are a dime a dozen. These are...
-
I spend half my time trying to figure out these accounting reports, said Paul Hribar in total frustration. He was trying to figure out whether to introduce a new product and was referring to the...
-
Patty Hayes owned four Personal Seat Licenses (PSLs) at the Cleveland Browns Stadium. Hayess PSLs reserved four seats on the 50-yard line, at the railing, on the north side of the stadium. As the...
-
At the end of the fiscal year, Siglo Delivery Services trial balance appeared as follows. Required 1. Enter the trial balance amounts in the Trial Balance columns of a work sheet and complete the...
-
Use the below table to answer the following questions. Selling Price $43.00 = Sales Volume Variable 2,200 3,200 Fixed Cost Cost 4,200 Profitability 5,200 6,200 $47,200 15 $14,400 $42,400 $70,400...
-
Write equilibrium expressions for each of the following equilibria. (a) 2 C(s) + O 2 (g) 2 CO(g) (b) Zn 2+ (aq) + H 2 S(g) ZnS(s) +2 H + (aq) (c) HCl(g) + H 2 O() H 3 O + (aq) + Cl (aq) (d) H 2...
-
Write equilibrium expressions for each of the following heterogeneous equilibria. (a) CaCO 3 (s) Ca 2+ (aq) + CO 3 2 (aq) (b) AgCl(s) Ag + (aq) + Cl (aq) (c) Mg 3 (PO 4 ) 2 (s) 3 Mg 2+ (aq) + 2...
-
How and when will you evaluate the success of the new product or service and its branding? Be sure to suggest processes that occur at regular intervals and extend across the business, explaining how...
-
Christopher notices that his Discover Card statement gives the company's Web address (Discovercard.com) and directs him there to pay his bills and answer his questions. What e-business strategy is...
-
3. How will you prepare and negotiate a contract?
-
Produce your weather table's complete output in Celsius rather than Fahrenheit Display all columns and in default order. All columns that deal with temperature need to display in Celsius Formula: ( 3...
-
Know how to choose different types of investment based on such factors as safety, risk, income, growth, and liquidity.
-
Using the 80% rule to determine the adequacy of an individuals liability coverage is extremely advantageous for homeowners because approximately 2 out of every 3 homes in the U.S. are underinsured....
-
A rotary lawnmower blade rotates at 3500 rev/min. The steel blade has a uniform cross section 1/8 in thick by 1 ¼ in wide, and has a ½-in-diameter hole in the center as shown in the...
-
Calculate I, , and a for a 0.0175 m solution of Na 3 PO 4 at 298 K. Assume complete dissociation. How confident are you that your calculated results will agree with experimental results?
-
The rectangular plate is deformed into the shape shown by the dashed lines. Determine the average normal strain along diagonal BD, and the average shear strain at corner B relative to the x, y axes....
-
The nonuniform loading causes a normal strain in the shaft that can be expressed as ε x = k sin (Ï/L x), where k is a constant. Determine the displacement of the center C and the...
-
The rectangular plate undergoes a deformation shown by the dashed lines. Determine the shear strain γ xy and γ x'y' at point A. y 0.01 in. 5 in. 0.02 in.-ES in.- -|-0.02 in....
-
(a) Consider a two-sector model Y = C+ I C 0.8Y1+100; I = 200; Find an expression for Y, when Y = 1700 (b) (10 Marks) (i) A firm's marginal cost function is given as: MC = Q + 2Q + 4. Find the total...
-
(c) Find the elasticity of output (Q) with respect to price (P) for the following functions and state whether the functions are elastic, inelastic or have unit elasticity. Q = 1/14 (11) Q = -10 + 2P...
-
(b) Find the marginal cost, the marginal revenue and the marginal product of as the case may be: TC = 4Q + 5Q + 18 (i) (ii) TR = 300-20 (iii) Q = 50K 1/3L2/3 Marks) at Q=5 at Q=10 (4 Marks) (4 Marks)...

Study smarter with the SolutionInn App


