Question: Using values from the table of standard reduction potentials, calculate the cell potentials of the following cells. (a) (b) (c) Fe(s) | Fe+ (aq) ||
Using values from the table of standard reduction potentials, calculate the cell potentials of the following cells.
(a) ![]()
(b) 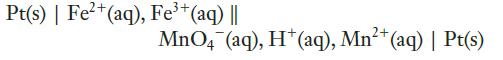
(c) ![]()
Fe(s) | Fe+ (aq) || Hg2+ (aq) | Hg()
Step by Step Solution
★★★★★
3.49 Rating (162 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

To calculate the cell potentials of the given cells using the standard reduction potentials we will ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


