A 25.0-mL sample of 1.44 M NH 3 is titrated with 1.50 M HCl. Calculate the pH
Question:
A 25.0-mL sample of 1.44 M NH3 is titrated with 1.50 M HCl. Calculate the pH at the equivalence point.
Choose an indicator from Table 16.4, and justify your choice.
Table 16.4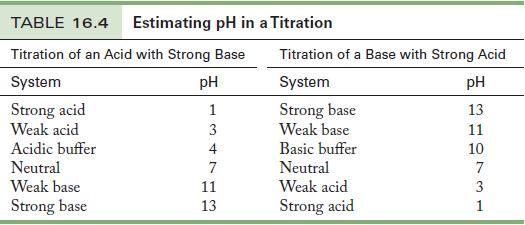
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
pH at the e...View the full answer

Answered By

Arun kumar
made more than four thousand assignments
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
A 10.0-mL solution of 0.300 M NH3 is titrated with a 0.100 M HCl solution. Calculate the pH after the following additions of the HCl solution: (a) 0.0 mL, (b) 10.0 mL, (c) 20.0 mL, (d) 30.0 mL, (e)...
-
Calculate the pH at the halfway point and at the equiva-lence point for each of the following titrations. a. 100.0 mL of 0.10 M HC7H5O2 (Ka = 6.4 105) titrated with 0.10 M NaOH b. 100.0 mL of 0.10 M...
-
Please write detailed roadmap/solution for all questions below. 1) An output of nmap search is shown below, a) Type the required terminal command and required parameters to obtain the shown output....
-
How do recruitment and selection practices contribute to high performance in an organization?
-
Predict the products of the following nucleophilic acyl substitutionreactions: (b) (a) NaOH NH3 H20 H (d) (c) Na* "OCH3 C CH3NH2 SCH3 "CH
-
From the financial managers perspective, describe the role of reorder points, safety stock, MRP, MRPII, and a just-in-time system in managing a firms inventory.
-
What factors have led to increased organizational interest in HR metrics and workforce analytics?
-
1. How is software adding value to automakers products? 2. How are the automakers benefiting from software- enhanced cars? How are customers benefiting? 3. What value chain activities are involved in...
-
Jargon Jen has five million shares outstanding, generates free cash flows of $12 million each year and has a cost of capital of 6%. It also has $3 million of cash on hand. Jargon Jen wants to decide...
-
Exactly 50 mL of a 0.0500 M solution of ethylamine, a base with K b = 1.1 10 -6 , is titrated with 0.100 M HNO 3 . What is the pH at the equivalence point? Suggest a good indicator from Table 16.4...
-
Chloropropionic acid, ClCH 2 CH 2 COOH, is a weak monoprotic acid with Ka = 7.94 10 -5 . Calculate the pH at the equivalence point in a titration of 10.00 mL of 0.100 M chloropropionic acid with...
-
Big Bull restaurant employs twenty-three employees who receive tips. During the current year, Big Bull has $410,000 in gross revenues, and its employees do not report any tip income. In what ways may...
-
Write a program that prints the numbers from 1 to 100. But for multiples of three, print "Fizz" instead of the number, and for the multiples of five, print "Buzz". For numbers that are multiples of...
-
In what ways do FDA regulations improve citizens' access to safer and more effective prescription drugs? In what ways does it hinder access? Why do government organizations that are designed to...
-
Analyze, critique and share your opinion on universal health care. Describe the principle in context of this policy issue. In what ways could access to health care be improved in a free market...
-
In depth discuss Stockman's analysis of the emergence of speculative or "bubble finance". Is Stockman accurate in his portrayal and if so, should we be concerned?How does this effect public policy?...
-
Write a function to determine whether a given string is a palindrome or not. Palindrome strings are those that read the same forwards and backwards.
-
Multiple Choice Question 1. The auditor wishes to gather evidence to test the assertion that the client's capitalization of leased equipment assets is properly valued. Which of the following sources...
-
A copper rod of length L =18.0 in is to be twisted by torques T (see figure) until the angle of rotation between the ends of the rod is 3.08. (a) If the allowable shear strain in the copper is 0.0006...
-
What size of standard hydraulic copper tube from Appendix G.2 is required to transfer 0.06 m 3 /s of water at 80C from a heater where the pressure is 150 kPa to an open tank? The water flows from the...
-
Determine the required size of new Schedule 80 steel pipe to carry water at 160F with a maximum pressure drop of 10 psi per 1000 ft when the flow rate is 0.5 ft 3 /s.
-
A device designed to allow cleaning of walls and windows on the second floor of homes is similar to the system shown in Fig. 11.20. Determine the velocity of flow from the nozzle if the pressure at...
-
A vital part of managing and administrating a large database is that of Database Backup and Database Recovery. Discuss this statement outlining the key issues. b)Discuss the potential of Cloud...
-
You find a referral left on your desk that outlines a patient who requires Diabetes Education due to a recent type 2 diagnosis after suffering a mild heart attack. You contact the emergency...
-
How many ways can a group of 7 adults and 4 children stand in a line if no two children are allowed to stand next to each other? How many numbers between 1 and 100 are multiples of 2, 3, or 6? How...

Study smarter with the SolutionInn App


