Chloropropionic acid, ClCH 2 CH 2 COOH, is a weak monoprotic acid with Ka = 7.94
Question:
Chloropropionic acid, ClCH2CH2COOH, is a weak monoprotic acid with Ka = 7.94 × 10-5. Calculate the pH at the equivalence point in a titration of 10.00 mL of 0.100 M chloropropionic acid with 0.100 M KOH. Choose an indicator from Table 16.4 for the titration.
Explain your choice.
Table 16.4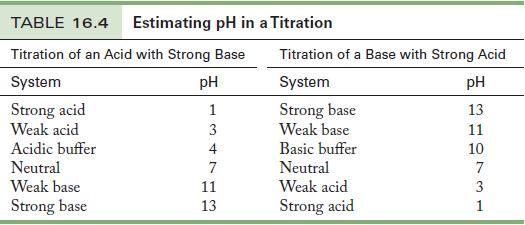
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
To calculate the pH at the equivalence point in a titration of a weak acid with a strong base we need to realize that at the equivalence point all the ...View the full answer

Answered By

Emel Khan
I have the ability to effectively communicate and demonstrate concepts to students. Through my practical application of the subject required, I am able to provide real-world examples and clarify complex ideas. This helps students to better understand and retain the information, leading to improved performance and confidence in their abilities. Additionally, my hands-on approach allows for interactive lessons and personalized instruction, catering to the individual needs and learning styles of each student.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Please write detailed roadmap/solution for all questions below. 1) An output of nmap search is shown below, a) Type the required terminal command and required parameters to obtain the shown output....
-
Ant venom contains formic acid (HCOOH; formica is the Latin word for ant). Suppose you are at a pharmaceutical company working on a quick antidote and need to estimate the pH at the stoichiometric...
-
What you thought you knew about the economics of healthcare and identify the significance (what it means to you professionally and/or personally). Please identify what more you want to learn in...
-
Describe Robertson Tool's business risk, making critical judgments. Consider the volatility of its revenues and operating expenses, therefore appraising the volatility of its EBIT. HINT: Consider the...
-
Rank the compounds in each of the following sets in order of their expected reactivity toward nucleophilic acylsubstitution: (a) , , , CH, HH. NH2 (b) CHC -CCI3, CHCICF3/2
-
A 2-kW electric resistance heater submerged in 5-kg water is turned on and kept on for 10 min. During the process, 300 kJ of heat is lost from the water. The temperature rise of water is (a) 0.4C (d)...
-
The optimum speeds (in kilometers per hour) for 30 hatchbacks Approximate the mean of the frequency distribution. Car Speeds (in kilometers per hour) Frequency 20-24 15 25-29 8. 30-34 4 35-39 3
-
Superior Micro Products uses the weighted-average method in its process costing system. Data for the Assembly Department for May appear below: Required: 1. Compute the cost per equivalent unit for...
-
2. Write a program in python to get the number from the user using GUI. Use 2 command buttons one to check whether the number is an amstrong number If the given number is a amstrong, display it is a...
-
A 25.0-mL sample of 1.44 M NH 3 is titrated with 1.50 M HCl. Calculate the pH at the equivalence point. Choose an indicator from Table 16.4, and justify your choice. Table 16.4
-
A chemist is developing a titration analysis for lactic acid. Lactic acid is a monoprotic acid with K a = 8.4 10 -4 . Calculate the pH at the equivalence point of a titration of 100 mL of 0.100 M...
-
Estimate E cell for the half reaction. 2H 2 O + 2e - H 2 + 2OH - given the following values of G o f : H 2 O(l) = 237 kJ/ mol H 2 (g) = 0.0 OH - (aq) = 157 kJ/ mol e - = 0.0 Compare this value of E...
-
A school friend who has joined a new company tells you that the company has many controls over cash. He asks you to explain why the following controls exist. a. Mail opening is carried out by two...
-
You are the senior accountant for a shoe wholesaler that uses the periodic inventory method. You have determined the following information from your companys records, which you assume is correct: a....
-
James Stewart owns a general store in a country town. He keeps two subsidiary ledgers (an accounts receivable ledger and an accounts payable ledger) and a general ledger. Balances in the subsidiary...
-
Sprintay Limited uses the balance sheet approach to account for its bad debts expense and provision for doubtful debts. Past experience indicates the following percentages of accounts receivable that...
-
List at least five techniques to successfully communicate with intercultural audiences orally and at least five tips for written messages. Be prepared to explain each.
-
What are the advantages and limitations of residual income (RI) as a performance measure?
-
To help you become familiar with the accounting standards, this case is designed to take you to the FASBs Web site and have you access various publications. Access the FASBs Web site at...
-
Turpentine at 77F is flowing from A to B in a 3-in coated ductile iron pipe. Point B is 20 ft higher than point A and the total length of the pipe is 60 ft. Two 90 long-radius elbows are installed...
-
Water at 15C is flowing downward in a vertical tube 7.5 m long. The pressure is 550 kPa at the top and 585 kPa at the bottom. A ball-type check valve is installed near the bottom. The hydraulic tube...
-
In a processing plant, ethylene glycol at 77F is flowing in a 6-in coated ductile iron pipe having a length of 5000 ft. Over this distance, the pipe falls 55 ft and the pressure drops from 250 psig...
-
Write a program that outputs the following. (Submit for 1 point). Hello world! (2) Update to output the following. (Submit again for 1 more point, so 2 points total). Hello world! How are you? (3)...
-
What does the predict() function of the sklearn KMeans class return?
-
with code examples and references for full credit. ArrayList and linked list are non-synchronized classes. Both are constructs that hold data, but each construct does so in a different way. Discuss...

Study smarter with the SolutionInn App


