Exactly 50 mL of a 0.0500 M solution of ethylamine, a base with K b = 1.1
Question:
Exactly 50 mL of a 0.0500 M solution of ethylamine, a base with Kb = 1.1 × 10-6, is titrated with 0.100 M HNO3. What is the pH at the equivalence point? Suggest a good indicator from Table 16.4 for this titration, and justify your selection.
Table 16.4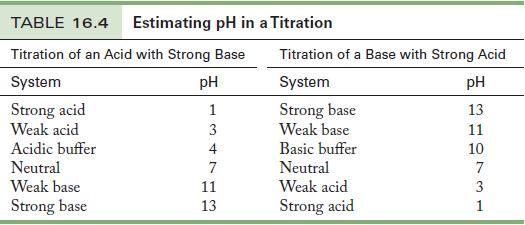
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To find the pH at the equivalence point you need to consider what happens when you titrate a weak base with a strong acid At the equivalence point all ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Sketch the titration curve from Problem 123 by calculating the pH at the beginning of the titration, at one-half of the equivalence point, at the equivalence point, and at 5.0 mL beyond the...
-
Please write detailed roadmap/solution for all questions below. 1) An output of nmap search is shown below, a) Type the required terminal command and required parameters to obtain the shown output....
-
Harry Bhel carries a business as a sole proprietorship. During its 2022 fiscal period, its first year of operations, the business had cash sales of $123,000. It also has sales on account of $46,000,...
-
The following structure represents tetrahedral alkoxide ion intermediate formed by addition of a nucleophile to a carboxylic acid derivative. Identify the nucleophile, the leaving group, the starting...
-
A frictionless pistoncylinder device and a rigid tank contain 2 kmol of an ideal gas at the same temperature, pressure, and volume. Now heat is transferred, and the temperature of both systems is...
-
The ages (in years) of the residents of a small town in 2012 Approximate the mean of the frequency distribution. Age (in years) Frequency 0-9 40 10-19 72 20-29 78 30-39 90 40-49 84 50-59 42 60-69 31...
-
Batch processes are often used in chemical and pharmaceutical operations to achieve a desired chemical composition for the final product and typically involve a transient heating operation to take...
-
A customer has a concern of slow cranking only on a hot restart. During the visual inspection, vou also notice the insulation on the negative cable is miched. What may cause this problem? a. The...
-
Write the chemical equilibria and expressions for the equilibrium constants for the ionizations of the following polyprotic acids. (a) Oxalic acid (b) Sulfurous acid
-
A 25.0-mL sample of 1.44 M NH 3 is titrated with 1.50 M HCl. Calculate the pH at the equivalence point. Choose an indicator from Table 16.4, and justify your choice. Table 16.4
-
In Exercises use a computer algebra system and the result of Exercise 77 to match the closed curve with its area. (a) (b) (c) (d) (e) (f) Data from in exercises 77 Use integration by substitution to...
-
The CFO of Olaf Limited has prepared a schedule based on her past experience indicating the following percentages of accounts receivable that have been written off as bad. The present balance of the...
-
With respect to the internal control over cash, provide an example of each of the following: a. Independent checks and reviews b. Approval of transactions c. Matching documents d. Prenumbering and...
-
Cardshark Ltd supplies boxes of Big Bash cricket cards to various card swap shops around Australia. The company has more than 100 small clients purchasing boxes of cards on credit. Credit terms are...
-
A university recently opened a parking station on its lower campus area for the benefit of the students. A guard has been engaged to patrol the car park and issue parking stickers to university...
-
Discuss the following statements: a. Internal control is the responsibility of the accountants in an organisation. b. A properly designed system of internal control over cash should prevent employee...
-
What is return on equity, how is it calculated, and how is it interpreted?
-
The population of Detroit, Michigan, decreased from 1,027,974 in 1990 to 688,701 in 2013 (Source: U.S. Census Bureau). Find the average rate of change in the population of Detroit, Michigan, over the...
-
For the system in Fig. 11.26, compute the total head on the pump and the power delivered by the pump to the coolant. Figure 11.26 4 ft Flow 20 GPM 4.0 ft 4.0 ft L- 30 ft 1.0 ft 10 GPM Flow 18 ft 2.0...
-
The tank shown in Fig. 11.24 is to be drained to a sewer. Determine the size of new Schedule 40 steel pipe that will carry at least 400 gal/min of water at 80 F through the system shown. The total...
-
Water at 60F is to flow by gravity between two points, 2 mi apart, at the rate of 13 500 gal/min. The upper end is 130 ft higher than the lower end. What size concrete pipe is required? Assume that...
-
Research to learn and then share what you believe are: the main components of an effective network security policy the potential issues caused by an ineffective or nonexistent policy three ways an...
-
If you are considering the possibility of adopting telecommunications for your company or upgrading them , it is important to take certain details into account. This way, you can be safer and avoid...
-
How does a Cisco network engineer use the OSI and TCP/IP models in real-world tasks?

Study smarter with the SolutionInn App


