Question: Although copper does not usually react with acids, it does react with concentrated nitric acid. The reaction is complicated, but one outcome is (a)
Although copper does not usually react with acids, it does react with concentrated nitric acid. The reaction is complicated, but one outcome is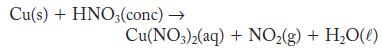
(a) Name all of the reactants and products.
(b) Balance the reaction.
(c) Assign oxidation numbers to the atoms. Is this a redox reaction?
(d) Pre-1983 pennies were made of pure copper. If such a penny had a mass of 3.10 g, how many moles of Cu are in one penny? How many atoms of copper are in one penny?
(e) What mass of HNO3 would be needed to completely react with a pre-1983 penny?
Cu(s) + HNO3(conc) Cu(NO3)2(aq) + NO(g) + HO()
Step by Step Solution
3.47 Rating (144 Votes )
There are 3 Steps involved in it
Lets go through each part of the question a Reactants and Products Reactants Copper Cu and Concentra... View full answer

Get step-by-step solutions from verified subject matter experts


