Are the compounds listed below soluble or insoluble in water? (a) Ba(NO 3 ) 2 (b) PbSO
Question:
Are the compounds listed below soluble or insoluble in water?
(a) Ba(NO3)2
(b) PbSO4
(c) LiOH
(d) AgCl
Strategy
Use the solubility rules in Table 4.1 to predict whether each compound is soluble or insoluble in water.
Table 4.1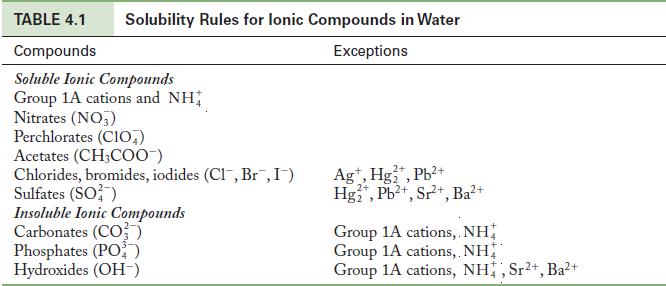
Transcribed Image Text:
TABLE 4.1 Compounds Soluble Ionic Compounds Group 1A cations and NH Nitrates (NO3) Solubility Rules for lonic Compounds in Water Exceptions Perchlorates (CIO) Acetates (CH3COO-) Chlorides, bromides, iodides (CI, Br, I) Sulfates (SO²) Insoluble Ionic Compounds Carbonates (CO3) Phosphates (PO) Hydroxides (OH-) 2+ Ag+, Hg₂+, Pb²+ Hg₂+, Pb²+, Sr²+, Ba²+ Group 1A cations,. NH Group 1A cations, NH Group 1A cations, NH, Sr²+, Ba²+
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a Table 41 indicates that all ionic compounds of the nitrate ion are soluble therefore BaNO ...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
According to FASB ASC Topic 830 (Foreign Currency Matters - Translation of Financial Statements), the appropriate method of restatement from a foreign currency to the U.S. dollar for each of the...
-
Use the solubility rules in Table 4.1 to predict whether BaSO 4 is soluble or insoluble in water. Table 4.1 TABLE 4.1 Compounds Soluble Ionic Compounds Group 1A cations and NH Nitrates (NO3)...
-
Apply the solubility guidelines in Table 5.1 to predict whether each of the following solids is water soluble or insoluble. For which are the solubility guidelines inconclusive? (a) Al 2 (SO 4 ) 3 ;...
-
Compute the 2-norm and o-norm of the following systems: 8+3 +1 (8+1)(8-2) 10 5 8+3 G(8) = G3(s) = -1 -21 1 0 0 2 3 0 01 7). [+] G(8) = 2 3 1 2 0 G4(8) = -1 1 0 1 0 -2 -3 0 1 10006 0 1 1 2 01 20 10 0 2
-
We lagged wire of length L and Cross-sectional area A has its ends maintained at temperatures T1 and T2. The thermal conductivity of the wire is given by K =B + CT Where T is the temperature and B...
-
If G is the group defined by generators a,b and relations a 2 = e, b 3 = e, then G Z 2 * Z 3 .
-
A circular current-carrying wire loop produces a magnetic field. A spinning disk of uniformly distributed charge produces a similar magnetic field. How would you expect the field due to a spinning...
-
On January 1, 2006, John Doe Enterprises (JDE) bought a 55% interest in Bubba Manufacturing, Inc. (BMI). JDE paid for the transaction with $3.5 million cash and 400,000 shares of JDE common stock...
-
1. How the singly linked lists can be represented? 2. How the doubly linked list can be represented? 3. What are benefits of ADT? 4. When singly linked list can be represented as circular linked...
-
During the courtship and negotiation stages, managers often emphasize "equal partnerships" and do not reveal (or even try to hide) their true intentions. What are the ethical dilemmas here?
-
A solution is formed by dissolving 3 g sugar in 100 mL water. Identify the solvent and the solute.
-
Although copper does not usually react with acids, it does react with concentrated nitric acid. The reaction is complicated, but one outcome is (a) Name all of the reactants and products. (b) ...
-
Journalize the following data taken from the payroll register of Himes Bakery as of June 12, 20--: Regular earnings $6,520.00 Overtime earnings.. 950.00 Deductions: Federal income tax. 782.00 Social...
-
Which mechanism of bacterial genetic transfer does not require recombination with the bacterial chromosome?
-
Identify the key events during meiosis that result in a 50% reduction in the amount of genetic material per cell.
-
Explain the difference between gene modification and gene addition. Is each of the following an example of gene modification or gene addition? A. A mouse model to study cystic fibrosis B....
-
Describe three distinct mechanisms for accomplishing dosage compensation in different species.
-
A human disease known as vitamin Dresistant rickets is inherited as an X-linked dominant trait. If a male with the disease produces children with a female who does not have the disease, what is the...
-
Krogstad Corporation bought 1,000 shares of Cole Inc. for $90 per share plus a brokerage fee of $1,800. Three months later, the shares were sold for $110 per share. The brokerage fee on the sale was...
-
You have just begun your summer internship at Omni Instruments. The company supplies sterilized surgical instruments for physicians. To expand sales, Omni is considering paying a commission to its...
-
Draw Haworth projections for cis-1,3-di-tert-butylcyclohexane and trans-1,3-di-tert butylcyclohexane. One of these compounds exists in a chair conformation, while the other exists primarily in a...
-
Identify the name of the parent for each of the following compounds: (a) (b) (c) (d)
-
Each of the structures in the previous problem has one or more substituents connected to the parent. (a) Identify the name of each substituent in 4.39a. (b) Identify the common name and the IUPAC...
-
Rev 004 Draft statements of financial position for Lion and Tau on 31 March 2020 are as follows: Lion Pula Tau Pula Non-current assets. 560,000 280,000 Property, plant and equipment 200,000 280,000...
-
1. 2. Concord purchased equipment on January 2, 2022, for $86,600. At that time, the equipment had an estimated useful life of 10 years with a $4,600 salvage value. The equipment is depreciated on a...
-
THE AM BAKERY Bakery Sales Actual Costs For the Month Ending November 30 Ingredients Flour Butter Oil Fruit Nuts Chocolate Actual $ 5,100 4,740 2,125 1,600 1,250 1,080 480 Other Total ingredients...

Study smarter with the SolutionInn App


