Question: Use the solubility rules in Table 4.1 to predict whether BaSO 4 is soluble or insoluble in water. Table 4.1 TABLE 4.1 Compounds Soluble Ionic
Use the solubility rules in Table 4.1 to predict whether BaSO4 is soluble or insoluble in water.
Table 4.1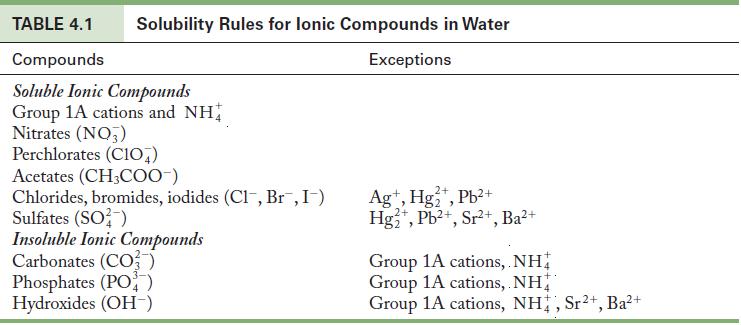
TABLE 4.1 Compounds Soluble Ionic Compounds Group 1A cations and NH Nitrates (NO3) Solubility Rules for lonic Compounds in Water Exceptions Perchlorates (C107) Acetates (CH3COO-) Chlorides, bromides, iodides (Cl, Br, I) Sulfates (SO) Insoluble Ionic Compounds Carbonates (CO3) Phosphates (PO4) Hydroxides (OH-) 2+ Ag+, Hg+, Pb+ Hg2+, Pb+, Sr+, Ba+ Group 1A cations, NH Group 1A cations, NH Group 1A cations, NH, Sr+, Ba+
Step by Step Solution
3.46 Rating (156 Votes )
There are 3 Steps involved in it
BaS... View full answer

Get step-by-step solutions from verified subject matter experts


