At 500 K, the equilibrium constant is 155 for H(g) + 1(g) 2HI(g) Calculate the equilibrium constant
Question:
At 500 K, the equilibrium constant is 155 for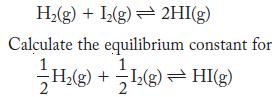
Transcribed Image Text:
H(g) + 1(g) 2HI(g) Calculate the equilibrium constant for HI(g) H(g) + (g) +1(g) =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Solution HI Kc 15...View the full answer

Answered By

User l_960928
I completed my degree in 2017. I am working as an Accountant.But I can manage all the subjects and I am expert in that.I did tuitions to some of the childrens up to plustwo.So I have the experience.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
(a) At 800 K the equilibrium constant for I2(g) 2 I(g) is Kc = 3.1 Ã 10-5. If an equilibrium mixture in a 10.0-L vessel contains 2.67 Ã 10-2 g of I(g), how many grams of I2 are in the...
-
Consider the equilibrium dissociation of carbon dioxide CO 2 CO + 1/2 O 2 . At 2500 K, the equilibrium constant is 0.03635. Calculate the enthalpy of reaction for this reaction at 2500 K and use...
-
At 700 K the equilibrium constant for the reaction Is Kp= 0.76. A flask is charged with 2.00 atm of CCl4, which then reaches equilibrium at 700 K. (a) What fraction of the CCl4 is converted into C...
-
Determine the resultant force and specify where it acts on thebeam measured from A . Assume F = 540 lb . Part A Determine the magnitude of theresultant force. Part B Determine the distance between A...
-
Only quantitative outcomes are relevant in capital budgeting analysis. Do you agree? Explain.
-
"The utility derived from consumption is intangible and unobservable. Therefore, the utility concept has no practical value. Discuss this statement.
-
Briefly describe the role of technology in conceptual, logical, and physical modeling.
-
Ferris Bueller, Inc., owns a building in Des Moines, Iowa, that was built at a cost of $5,000,000 in 2000. The building was used as a manufacturing facility from 2001 to 2010. However, economic...
-
(No program is required for this problem.) Simpson's rule for numerical integration [f(x)dx] approximates a function f(x) (blue curve in the figure) by a parabola, f(x) = ax + Bx+y, within two...
-
If 200 mL of 0.010 M CaCl 2 (aq) is mixed with 300 mL of 0.150 M NaOH(aq), will Ca(OH) 2 precipitate?
-
At 2000 K, experiments show that the equilibrium constant for the formation of water is 1.6 10 10 . 2H(g) + O(g) 2HO(g) Calculate the equilibrium constant at the same tempera- ture for H(g) + O(g) =...
-
Zarson's Netballs is a manufacturer of high-quality basketballs and volleyballs. Setup costs are driven by the number of setups. Equipment and maintenance costs increase with the number of...
-
To maximize net income, the return on the interest-bearing account would have to be greater than the net revenue from the rental property. The net revenue from the rental property is the revenue...
-
At the end of 2021, India's weak currency, the rupee, was showing a high trade deficit due to higher imports and slow growth in exports. Amazon has tried for several years to enter the e-commerce...
-
As you can see from Lesson 3, our perceptions of issues are influenced by many factors. All of us, at one time or another, have judged others and formed some level of discrimination. There are three...
-
Discuss the power of reasoning to help solve such issues: Is there research (or potential research) that could answer the relevant questions? What can we do to think more objectively about solutions...
-
A policy framework is a model for systemically examining a specific social welfare policy or a set of policies (Karger & Stoez, 2022). These can be used by analysts to analyze data on a policy a...
-
Goldman Sachs believes that market volatility will be 20% annually for the next 3 years. Three-year at-the-money call and put options on the market index sell at an implied volatility of 22%. What...
-
What is a lobbyist in US? How did this term emerge?
-
A person stands on level ground and throws a baseball straight upward, into the air. (a) Does the person exert a force on the ball while it is in his hand? After it leaves his hand? (b) Does the ball...
-
Figure P2.15 shows several hypothetical positiontime graphs. For each graph, sketch qualitatively the corresponding velocity time graph. Figure P2.15 Case 1 Case 2 Case 3
-
Figure Q2.15 shows a motion diagram for a rocket powered car. The photos are taken at 1.0-s intervals. Make qualitative plots of the position, velocity, acceleration, and force on the car as...
-
Using the the table of possible parameters below, a student was asked to determine an equation in the form of y=ab(x-h) +k for a radical function that has a domain of { |-2, zeR} and a range of {y y
-
The load L, in pounds, on a certain sail varies directly as the square of the wind speed v, in miles per hour. If the load on a sail is 560 lb when the wind speed is 20 mph, what is the load on the...
-
An online math tutor service would like to claim that the average time spent taking a standardized test after students go through tutoring is less than the average time spent before going through...

Study smarter with the SolutionInn App


