Calculate the equivalence-point volume in the titration of 50.00 mL of 0.0884 M nitric acid with 0.0980
Question:
Calculate the equivalence-point volume in the titration of 50.00 mL of 0.0884 M nitric acid with 0.0980 M NaOH. The titration curve for this system appears in Figure 16.2.
Strategy
Use the concentration and volume of the acid (both given) to calculate the number of moles of acid. Then use the stoichiometry to calculate the number of moles of base needed to react with the acid. Last, use the concentration of base (given) as a conversion factor to calculate the volume of base needed.
Figure 16.2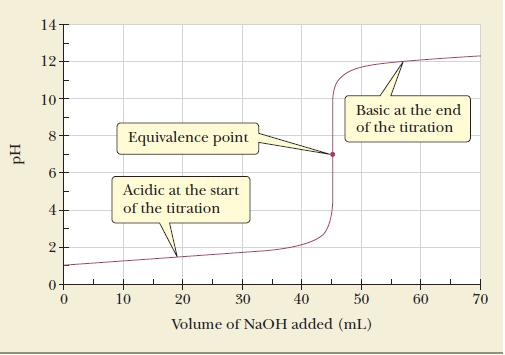
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





