Calculate the potential for each of the voltaic cells in Exercise 18.44 when the concentrations of the
Question:
Calculate the potential for each of the voltaic cells in Exercise 18.44 when the concentrations of the soluble species and gas pressures are as follows:![(a) [Fe+] = 0.050 M, [Zn+] = 1.0 10- M (b) [Fe+] = 0.20 M, [Fe+] = 0.010 M, [CI-] = 4.0 X 10-3 M (c) [Br] =](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/7/1/1/350659bd4b6b819a1704711350729.jpg)
Exercise 18.44
For each of the reactions, calculate E° from the table of standard potentials, and state whether the reaction is spontaneous as written or spontaneous in the reverse direction under standard conditions.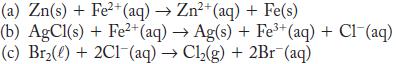
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Calculate the potential for each of the voltaic cells in Exercise 18.43 when the concentrations of the soluble species and gas pressures are as follows: Exercise 18.43 Use the standard reduction...
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
Calculate the standard cell potential for each of the electrochemical cells in Problem 44. Problem 44 Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the...
-
You are a lead auditor from ABC Auditors Pty Ltd. Your client is Cassey's Quality Cars Ltd who are a large car dealership with branches across Queensland. ABC Auditors have performed audits for past...
-
It is known that on clear nights a thin layer of water on the ground will freeze before the air temperature drops below 0C. Consider such a layer of water on a clear night for which the effective sky...
-
Refer to Data Set 22 in Appendix B for the axial loads of aluminum cans that are 0.0111 in. thick. The load of 504 lb is an outlier because it is very far away from all of the other values. Construct...
-
How does architecture relate to design?
-
How do they relate to the difference between tax expense and taxes payable? How could an organization have a tax receivable? Why is the tax expense reported on the income statement comprised of...
-
(5 points) A memory hierarchy has following hit rates and average access time of CPU. Calculate the last hit rate. Hit Rate Average Access Time of CPU (ns) 0.75 25 0.85 20 ??? 15
-
A voltaic cell consists of a lead electrode and a reference electrode with a constant potential. This cell has a voltage of 53 mV when the lead electrode is placed in a 0.100 M Pb(NO 3 ) 2 solution...
-
Disproportionation is a type of redox reaction in which the same species is simultaneously oxidized and reduced. One species that undergoes disproportionation is Cu + (aq).
-
Name and describe the five primary bond variables that can be solved using Excel spreadsheets.
-
Suppose that on December 31 of the current year, Jones Company reported long-term investments of $20,000. After careful evaluation, Jones concluded that the market value of the investments had...
-
Define your personal Gross National Product(GNP), by forcasting your expenses for the year. Categorize each of your expenses into one of 3 categories, Consumption, Savings, or Government Spending...
-
ABC Company had the following transactions during July. 1-Jul Beginning inventory 100 units $80 each 2-Jul Purchase 120 units $82 each 10-Jul Sale 90 units 13-Jul Sale 50 units 15-Jul Purchase 50...
-
An 20-year annuity-immediate has monthly payments of $400 and a present value of $36000 Find the effective annual interest rate.
-
Bayside Fishing Supply Co . is acquiring Fishing Lure Specialists for $ 2 5 , 2 5 0 in cash. Bayside Fishing Supply C 0 . has 2 , 3 5 0 shares outstanding at a market price per share of $ 3 9 ....
-
What important control functions are served by mailing monthly statements to customers? Why is it important to separate the duties of responding to customer complaints from the accounts receivable...
-
What are current assets and current liabilities? How are they different from non-current assets and non-current liabilities?
-
Work problem 5.73 again, but this time the camera is to sit atop the float, out of the water. What minimum diameter float is required for this arrangement? If the float is made of a larger diameter,...
-
The diving bell shown in Fig. 5.2 weighs 72 kN and has a volume of 6.5 m 3 . Find the tension in the cable when the sub is (a) Hanging above the water, (b) Once the sub is lowered into the sea water....
-
Convert a volume flow rate of 3.0 gal/min to m 3 /s.
-
Define the major items on the assets and liabilities sides of a banks balance sheet. If a bank has $100 million in assets and $80 million in liabilities, what is the banks net worth? What is credit...
-
Zero rates for one year, two year and 3 year investments are 4%, 5% and 5.6% per annum with continuous compounding. What is the forward rate for year 3 (% per annum with continuous compounding)?
-
Gavaskar of Bombay sent 100 TV sets to Kapil of Chandigarh on consignment basis. The cost price of each set is ~ 5,000. Gavaskar paid ~ 100 for cartage, ~ 1,500 for railway freight and ~ 400 for...

Study smarter with the SolutionInn App


