Calculate the standard potential and state the direction in which the reaction proceeds spontaneously for Strategy Find
Question:
Calculate the standard potential and state the direction in which the reaction proceeds spontaneously for![]()
Strategy Find the two half-reactions in Table 18.1, reversing one of them to make it an oxidation process. Combine the E°s for the two half-reactions to determine the voltage of a voltaic cell having the above reaction.
Table 18.1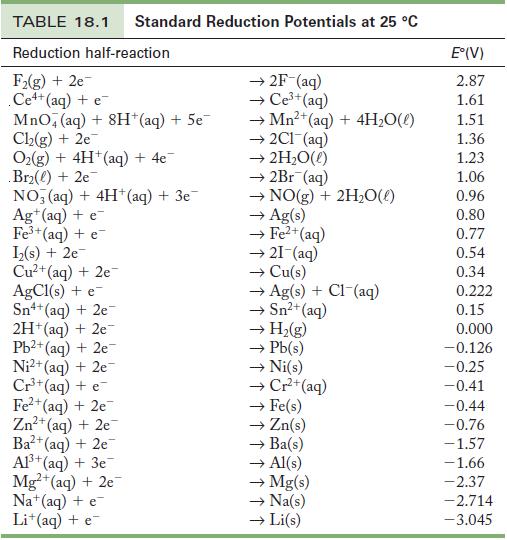
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
From Table 181 The top halfreaction must be reversed and ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
A client invested $100 in a mutual fund at the start of the month. After 20 days, the portfolio gained 10% (i.e., value = $110), and the client added an extra $50 (total portfolio value=$160). From...
-
The seal of a reservoir is described by h(x, y) = -5 x 10-8 (3x + 6y) ft for the range of x = [-500, 500] ft and y = [-500, 500] ft. All of the volume underneath this seal acts as a condensate...
-
For the current fiscal year, Purchases were $187,000, Purchases Returns and Allowances were $4,200 and Freight In was $10,500. If the beginning merchandise inventory was $98,000 and the ending...
-
An assistant treasurer is currently reevaluating their firm's banking relationship. The firm's current lender charges an effective borrowing cost of 4.25 percent. A competing lender provides the...
-
The spectral transmissivity of plain and tinted glass can be approximated as follows: Plain glass: = 0.9 0.3 < < 2.5m Tinted glass: = 0.9 0.5 < < 1.5m Outside the specified wavelength ranges, the...
-
Benzene and toluene form nearly ideal solutions. The boiling point of pure benzene is 80.1*C, Calculate the chemical potential of benzene relative to that of pure benzene when xbmzenc = 0.30 at its...
-
True or False. It is best to provide a customer with every piece of recorded data for a final report.
-
Williams Corporation imports, from a number of German manufacturers, large machining equipment used in the tooling industry. On June 1, the company received delivery of a piece of machinery with a...
-
Create a MATLAB script that generates a scatter plot for the given data points and labels the axes appropriately. x = [ 1 , 2 , 3 , 4 , 5 ] y = [ 2 , 4 , 1 , 3 , 5 ] The data points are provided in...
-
Use Table 18.1 to (a) List metals that are and are not oxidized by H + (aq) under standard conditions. (b) Find an oxidizing agent that will oxidize copper metal. Strategy Consult Table 18.1 as an...
-
Consider the cell shown in Figure 18.3. One half-cell consists of silver metal in a silver nitrate solution, and the other half-cell has a piece of copper metal immersed in a copper(II) nitrate...
-
Another model for diffusion is called the Bernoulli-Laplace model. Two urns (urn A and urn B) contain a total of 2k molecules. In this case, k of the molecules are of one type (called type I...
-
How is the auditor of a government company appointed?
-
What are the points you will consider at the time of examining the issue of right shares?
-
Describe in brief the prudential norms of RBI relating to advances.
-
Information and means of information are by no-means equivalent terms. Comment.
-
An auditor is not a valuer, though he is intimately connected with values. Discuss referring to the relevant case decisions.
-
This problem can be used in conjunction with Problem 2-58A. Refer to Problem 2-58A. Requirements 1. Journalize the June transactions of Martin Resources, Inc. Explanations are not required. 2....
-
Complete problem P10-21 using ASPE. Data from P10-21 Original cost ................................................................. $7,000,000 Accumulated depreciation...
-
Compute the time required to reduce the depth in the tank shown in Fig. 6.14 by 225 mm if the original depth is 1.38 m. The tank diameter is 1.25 m and the orifice diameter is 25 mm. dh
-
Compute the time required to reduce the depth in the tank shown in Fig. 6.14 by 1.50 m if the original depth is 2.68 m. The tank diameter is 2.25 m and the orifice diameter is 50 mm. dh
-
Compute the time required to empty the tank shown in Fig. 6.14 if the original depth is 18.5 in. The tank diameter is 22.0 in and the orifice diameter is 0.50 in. dh
-
What benefits and drawbacks are there for a business that uses a Standard/Traditional Costing model? What benefits and drawback are there for a business that uses an Activity Based Costing (ABC)...
-
Suppose that Bob's utility function is uB(qB,mB) = mB. (a) What is Bob's optimal consumption bundle if his income is Y, the price of qB is pq and the price of mg is 1. Ann's utility function is the...
-
Chandler has seventeen photos of his friends. In how many ways can he choose eleven of the photos to add to his photo album?

Study smarter with the SolutionInn App


