Consider the cell shown in Figure 18.3. One half-cell consists of silver metal in a silver nitrate
Question:
Consider the cell shown in Figure 18.3. One half-cell consists of silver metal in a silver nitrate solution, and the other half-cell has a piece of copper metal immersed in a copper(II) nitrate solution. A salt bridge that contains a sodium nitrate solution connects the two half-cells. Th e measured voltage is positive and the electrons fl ow in the direction shown in the diagram.
(a) Write the half-reaction that occurs in each half-cell.
(b) Write the equation for the overall chemical change that takes place.
(c) Identify the anode and the cathode.
(d) Indicate which half-cell is the negative electrode and which half-cell is the positive electrode.
(e) Indicate the direction the nitrate ions flow through the salt bridge.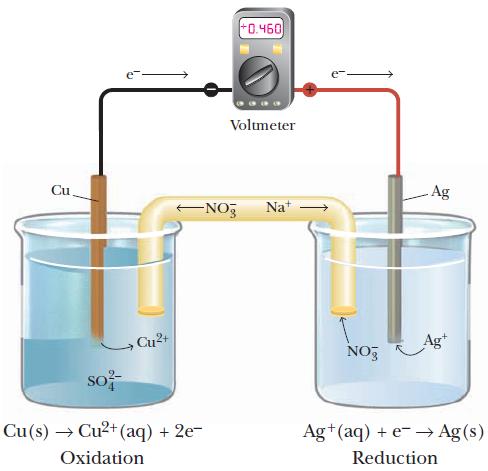
Strategy
Use the definitions and conventions introduced in the text to identify the various parts of the voltaic cell and their functions.
Step by Step Answer:

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball





