Question: A chemist is developing a titration analysis for lactic acid. Lactic acid is a monoprotic acid with K a = 8.4 10 -4 .
A chemist is developing a titration analysis for lactic acid. Lactic acid is a monoprotic acid with Ka = 8.4 × 10-4. Calculate the pH at the equivalence point of a titration of 100 mL of 0.100 M lactic acid with 0.500 M NaOH. Suggest an indicator from Table 16.4, and explain why you chose it.
Table 16.4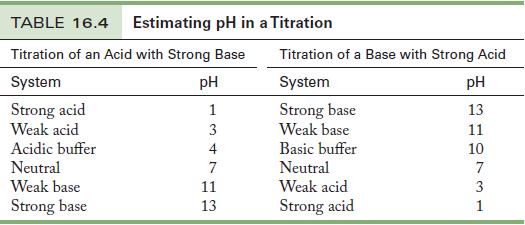
Step by Step Solution
3.36 Rating (149 Votes )
There are 3 Steps involved in it
pH at the endpoin... View full answer

Get step-by-step solutions from verified subject matter experts


