Determine the entropy change when 1 mol H 2 O boils at its normal boiling point of
Question:
Determine the entropy change when 1 mol H2O boils at its normal boiling point of 100.0 °C . Th e heat of vaporization of water is 44.0 kJ/mol .
Strategy
We will use Equation 17.5, remembering that when the temperature variable is used in this equation, it must be in kelvin units.
Equation 17.5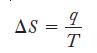
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Because we have 1 mol H 2 O the heat i...View the full answer

Answered By

AJIN kuriakose
I have completed B.Tech in Electrical Engineering & Masters in Power & Control From one of the best universities in India. I got the 99.05 percentile in the Gate Electrical Engineering Exam. I can Help students solving assignments in Electrical subjects like Power Electronics, Control system, Analog, Network Theory & Engineering Mathematics. Clear your fundamentals and develop problem-solving skills and analytical skills to crack the exam.
Get guidance and the opportunity to learn from experienced...
I can provide tuition for Electrical engineering subjects (Power Electronics, Digital electronics, Network Theory, Control System & Engineering Mathematics). The toughest subject of Electrical engineering can be made simple in online classes...
I can also solve it.
1 .I can help you with your assignments or exams or quiz or tutoring.
2. Very strict to the deadlines.
Message me for any help in assignments, live sessions. I am here to help students for all assignments, tests and exams and I will make sure you always get _95% In your subject.
Contact me in solution inn for any help in your semester, projects and for many more things . Also feel free to contact me through solution inn and for any advise related to tutoring and how it works here.thank you.
5.00+
5+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
The enthalpy of vaporization of methanol is 35.27 k] mol-I at its normal boiling point of 64.1oC. Calculate (a) The entropy of vaporization of methanol at this temperature and (b) The entropy change...
-
Determine the entropy change when 1 mol H 2 O freezes at its normal freezing point of 0.0 C. Th e heat of fusion of water is 6.01 kJ/mol.
-
The normal boiling point of Br2(l) is 58.8C, and its molar enthalpy of vaporization is Hvap = 29.6 kJ/mol. (a) When Br2(l) boils at its normal boiling point, does its entropy increase or decrease?...
-
Explain factors governing recruitment?
-
Tert-Butoxycarbonyl azide, a reagent used in protein synthesis, is prepared by treating tert-Butoxycarbonyl chloride with sodium azide. Propose a mechanism for thisreaction. , H3C CH3 + NaN3 NacI ...
-
Consider the following code using the POSIX Pthreads API: thread2.c #include #include #include #include int myglobal; void *thread_function(void *arg) { int i,j; for ( i=0; i <20; i++ ) { j=myglobal;...
-
For a Markov process define its infinitesimal operator: \[T(t) f\left(X_{s} ight)=\int f(y) p\left(t, X_{s}, y ight) d y=E\left[f\left(X_{t+s} ight) \mid X_{s} ight]\] Show that this operator is a...
-
Guardian Security Services was established on August 15, 2010, to provide security services. The services provided during the remainder of the month are listed below. Aug. 18. Issued Invoice No. 1 to...
-
Remarks: Describe a convention for a hub to find out about its nearby neighbors. You ought to indicate the arrangement of your messages and the size of any message fields. [4 marks] Using the...
-
Calculate the standard entropy change when 1 mol of propane burns in oxygen. Strategy Use the standard molar entropies from Appendix G. For each product and reactant, do not forget to multiply the...
-
E is -2.21 10 3 kJ/mol for the combustion of propane. Calculate H for this reaction at 25 C and 1.00 atm . Strategy Use Equations 17.3 and 17.4 to determine H. The change in the number of moles of...
-
Refer to the data in PE 14-21. Calculate Pace Companys average collection period. Accounts receivable balance, December 31 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . $ 60,000...
-
How is the Australian cereal market segmented? In other words, what groupings of customers are there? You will need to ask yourself who are the key customers for each of the segments?
-
Why do I have to know how a bill becomes a law? Why is common law important? Discuss the significance of common laws for the healthcare professional and the differentiate between common law and...
-
1.What do you understand by common law? (25%) 2. What are some advantages and disadvantages of the common law system? (25%) 3. Take a case example where both statue law and common law were applied....
-
Provide 5 questions in each area of S, P, I & N on a cell phone. You will use these at your discovery appointment, so make them good. In this meeting you will be asking these questions to your client...
-
1. Under traditional common law, in accepting an offer, may an offeree requestadditional terms? 2. What differences does the UCC make to the common law "mirror image" rule?
-
What are the advantages and disadvantages of using a questionnaire compared to a flowchart for documenting and assessing internal control?
-
How can you tell from the vertex form y = a(x - h) 2 + k whether a quadratic function has no real zeros?
-
For the flow of water in the square tubes described in Problem 9.32, compute the pressure drop over a length of 22.6 m. All surfaces are copper and the duct is horizontal.
-
For the glycerin described in Problem 9.31, compute the pressure drop for a horizontal duct 22.6 m long. All surfaces are copper.
-
For the heat exchanger described in Problem 9.29, compute the pressure drop for a length of 57 in.
-
Using the 3 Ps of resilience, (Permanence, Personalization, Pervasiveness) think of a difficult time or situation you have gone through. Provide your response to the questions below. Applying your...
-
Let f(x) = ax2 + bx + c, where a, b, and c are elements of the field F. Let d EF. Let a2, ai, and ao be the coefficients of the powers of x -- d in the representation f(x) = a2(x d)2 + ai(x d) + ao....
-
n architect makes a model of a new house with a patio made with pavers. In the? model, each paver in the patio is23in.long and12in.wide. The actual dimensions of the pavers are shown. Complete partsa...

Study smarter with the SolutionInn App


