Explain why the lattice energy of Na 2 O is considerably greater than that of NaF. Strategy
Question:
Explain why the lattice energy of Na2O is considerably greater than that of NaF.
Strategy
Lattice energies are evaluated by using the relationship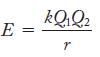
In general, the changes that can take place with the Q terms are more important than small changes in r.
Transcribed Image Text:
E = kQ1 Q₂ r
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The lattice energy of Na 2 O is greater because the greater charge on ...View the full answer

Answered By

Leah Muchiri
I am graduate in Bachelor of Actuarial Science and a certified accountant. I am also a prolific writer with six years experience in academic writing. My working principle are being timely and delivering 100% plagiarized free work. I usually present a precised solution to every work am assigned to do. Most of my student earn A++ GRADE using my precised and correct solutions.
4.90+
52+ Reviews
125+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Explain why the lattice energy of Na 2 O is considerably greater than that of NaF. Strategy Lattice energies are evaluated by using the relationship In general, the changes that can take place with...
-
Explain why the lattice energy of Na 2 O is considerably greater than that of NaF. Strategy Lattice energies are evaluated by using the relationship In general, the changes that can take place with...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
How many orders of magnitude is 3 . 2 \ times 1 0 - 9 m smaller than 0 . 0 0 0 0 4 m ?
-
The table below gives experimental results for a measurement of the period of motion T of an object of mass m suspended on a spring versus the mass of the object. These data are consistent with a...
-
Walker, Inc., has the following capital structure: Preferred stock $25 par value, 10,000 shares authorized, 7,000 shares issued and outstanding . . . . . . . . . . . . . . . . . . . . . . . . . . . ....
-
Stability of flow in torsional flow. Taylor determined the critical speed of rotation for flow between concentric cylinders with the inner cylinder rotating. The transition is characterized by a...
-
First American Bank is trying to determine whether it should install one or two drive-through teller windows. The following probability distributions for arrival intervals and service times have been...
-
Suppose you borrowed $12,000 at a rate of 9.6% and must repay it in 5 equal installments at the end of each of the next 5 years. How much interest would you have to pay in the first year?
-
Use Lewis electron-dot symbols to show the electron transfer during the formation of each compound from the appropriate atoms. (a) Beryllium oxide (b) Yttrium chloride
-
Write the Lewis structure of dimethyl ether, CH 3 OCH 3 .
-
An order for 100 of a product is processed on operation A and operation B. The setup time on A is 50 minutes, and the run time per piece is 9 minutes. The setup time on B is 30 minutes, and the run...
-
Ch. 21 - Final Exam > Pg. 4 - Course Final Exam GET $25 ibri Real Estate X 6 of 100 Roger has been farming his land in Florida for nearly 30 years. His principal crops are corn and soybeans. He also...
-
You have just been appointed to the board of a small dedicated emerging market asset manager with total assets under management (AUMs) of GBP250 million pounds, Tiger Investment Management, which...
-
Develop an interview plan. Include pre-interview preparations, interview outline, etc. Please note: Do not state what you are going to do but develop an actual interview plan from beginning...
-
Now derive the transient (time-dependent) velocity profile for pulsatile flow between parallel v plates. [note that now the governing equation has changed to include time dependence p at P Assume...
-
Google eagerly relies on machines as opposed to individuals since machines are coordinated correspondingly which gives individuals heaps of decisions to look at. This decision given by the machine is...
-
True or False: 1. There is virtually no positive correlation between the earnings of grandparents and grandchildren. 2. Other things being equal, workers who prefer more amenities at work or more...
-
Which one of the following anhydrous chloride is not obtained on direct heating of its hydrated chloride? (A) BaCl2 (B) CaClz (C) MgCl2 (D) SrCl2
-
Draw a bond-line structure for each of the following amino acids. (a) l-Leucine (b) l-Tryptophan (c) l-Methionine (d) l-Valine
-
Although most naturally occurring proteins are made up only of l amino acids, proteins isolated from bacteria will sometimes contain d amino acids. Draw Fischer projections for d-alanine and...
-
Compound A is a d-aldopentose. When treated with sodium borohydride, compound A is converted into an alditol that exhibits three signals in its 13C NMR spectrum. Compound A undergoes a...
-
Can you think of a real-world situation where a high central tendency measure masked significant dispersion in public opinion, leading to unexpected outcomes or challenges for a political leader or...
-
Use the Vigenre cipher with the keyword 'optical' to encrypt the following sentence: "Computer skills". Show your work in details in the form of table. Table below help you to find the code...
-
To configure a switch to which a certain amount of time before automatically recovering from a port security error use what command?
Master The Basics Of Ripple Cryptocurrency Investment 1st Edition - ISBN: 979-8868101717 - Free Book

Study smarter with the SolutionInn App


