Identify the intermolecular forces of attraction, and predict which substance of each pair has the stronger forces
Question:
Identify the intermolecular forces of attraction, and predict which substance of each pair has the stronger forces of attraction.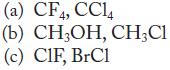
Strategy
All of the molecules will have London dispersion forces. We must determine whether a molecule is polar to determine whether dipole-dipole forces exist. If a hydrogen atom is bonded to an N, O, or F, hydrogen bonding will exist.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





