Calculate the energy necessary to boil 100.0 g carbon disulfide, CS 2 , at its normal boiling
Question:
Calculate the energy necessary to boil 100.0 g carbon disulfide, CS2, at its normal boiling point.
Strategy
Determine the number of moles of carbon disulfide; then use the enthalpy of vaporization from Table 11.3 as a conversion factor to determine the energy needed.
Table 11.3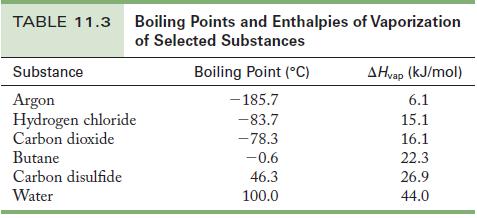
Transcribed Image Text:
TABLE 11.3 Boiling Points and Enthalpies of Vaporization of Selected Substances Substance Argon Hydrogen chloride Carbon dioxide Butane Carbon disulfide Water Boiling Point (C) -185.7 -83.7 -78.3 -0.6 46.3 100.0 AHvap (kJ/mol) 6.1 15.1 16.1 22.3 26.9 44.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
The molar mass of CS 2 is 7613 gmol The number o...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Table B.2 of Appendix B provides parameters for an equation that gives P sat as a function of T for a number of pure compounds. For one of them, determine the heat of vaporization at its normal...
-
The enthalpy of vaporization of methanol is 35.27 k] mol-I at its normal boiling point of 64.1oC. Calculate (a) The entropy of vaporization of methanol at this temperature and (b) The entropy change...
-
The normal boiling point of Br2(l) is 58.8C, and its molar enthalpy of vaporization is Hvap = 29.6 kJ/mol. (a) When Br2(l) boils at its normal boiling point, does its entropy increase or decrease?...
-
Determine which of the following statement(s) will always be true. It is possible that more than one statement is true. Only write down the letters of the statements that are true. n +1 2.1 The...
-
In the circuit in figure, the switch moves from position 1 to position 2 at t = 0. Use Laplace transforms to find v(t) for t >0. 6 t= 0 12 v (+ 100 pF (1)a 6 kn
-
Speedy Swift is a package delivery service that serves the greater Atlanta, Georgia, metropolitan area. To maintain customer loyalty, one of Speedy Swifts performance objectives is on-time delivery....
-
Consider the vector \(u\) that consists of 32 equally spaced samples of the function \(f(t) \approx \cos (4 \pi t)\) on the interval [0,1]. That is, \(u_{1}=f(0), u_{2}=\) \(f\left(\frac{1}{32}...
-
Flexible budget (Refer to data in Exercise 7-26). Suppose the static budget was for 2,500 units of output. Actual output was 2,000 units. The variances are shown in the following report: What are the...
-
What would be the duration of the zero-coupon bond with 18 months to maturity, face value of $5,000,000, and a yield of 7.55% p.a.?
-
Aspartame is a compound that is 200 times sweeter than sugar and is used extensively (under the trade name NutraSweet) in diet soft drinks. The skeleton structure of the atoms in aspartame is H-0. ....
-
Identify the intermolecular forces of attraction, and predict which substance of each pair has the stronger forces of attraction. Strategy All of the molecules will have London dispersion forces. We...
-
Complete Inventory Table B for the total retail value.
-
Post a brief explanation of your critical question. Then, synthesize the 4-5 articles you identified that address your critical question. Using the same language you would use with stakeholders,...
-
Bob, a customer service manager at a call center, prides himself on being able to "read" people. He says he can tell whether someone will make a good employee as soon as he meets them and "sizes"...
-
Thoughts on Responding: Do you think evaluations are fair for both students and instructors? Do they or can they capture the performance properly? In what ways is your performance as a student...
-
How can telemedicine programs reduce health disparities and inequalities for patients in underserved areas of the community or of lower economic status? Despite the technological advances in health...
-
Berger points out that "While we may be overloaded, we are also gaining increased control." In his example, the VCR gave people in the 80s the ability to choose when they would consume a television...
-
Three employees in the maintenance department are responsible for repairing the video games at Pinball Wizard, a video arcade. A maintenance worker can fix one video game machine every B hours on...
-
Write a program to move a signed number from smaller register to bigger register. Hint: movzx ax, bl Topic: Data Related Operators and Directives in assembly language
-
The inner ring A has an inner radius r 1 and outer radius r 2 . The outer ring B has an inner radius r 3 and an outer radius r 4 , and r 2 > r 3 . If the outer ring is heated and then fitted over the...
-
The ring, having the dimensions shown, is placed over a flexible membrane which is pumped up with a pressure p. Determine the change in the inner radius of the ring after this pressure is applied....
-
An A-36-steel hoop has an inner diameter of 23.99 in., thickness of 0.25 in., and width of 1 in. If it and the 24-in.-diameter rigid cylinder have a temperature of 65° F, determine the...
-
10. Your employer contributes $100 a week to your retirement plan. Assume that you work for this employer for another 14 years and the applicable discount rate is 7.25%. Given these assumptions, what...
-
You have just started a new job and are thrilled to learn that your new employer offers a 401(k) retirement plan to its employees. Your annual salary is $90,000. Assume the IRS allows you to...
-
1. Define Trade union and explain the functions of the Trade union? As a Manager do you Support Trade unions at workplace. 2. What are the different stages of Trade union Development? Discuss in...

Study smarter with the SolutionInn App


