Molecular nitrogen can be converted to NO in two steps. The equations follow. These two reactions are
Question:
Molecular nitrogen can be converted to NO in two steps. The equations follow. These two reactions are the first steps in the industrially important conversion of nitrogen into nitric acid. Calculate the mass of NO formed from 100 g N2 and excess H2 and O2.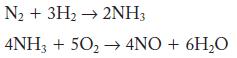
Transcribed Image Text:
N₂ + 3H₂ → 2NH3 4NH3 + 50₂ → 4NO + 6H₂O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
2...View the full answer

Answered By

Sidharth Jain
My name is Sidharth. I completed engineering from National Institute of Technology Durgapur which is one of the top college in India. I am currently working as an Maths Faculty in one of the biggest IITJEE institute in India. Due to my passion in teaching and Maths, I came to this field. I've been teaching for almost 3 years.
Apart from it I also worked as an Expert Answerer on Chegg.com. I have many clients from USA to whom I teach online and help them in their assignments. I worked on many online classes on mymathlab and webassign. I guarantee for grade 'A'.
4.90+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Various uses for nitric acid are given in Problem 6.43, along with information about how this important chemical is synthesized industrially. The key reactions are oxidations of ammonia to nitric...
-
Nitric acid is a chemical intermediate primarily used in the synthesis of ammonium nitrate, which is used in the manufacture of fertilizers. The acid also is important in the production of other...
-
The source of oxygen that drives the internal combustion engine in an automobile is air. Air is a mixture of gases, principally N2 (~79%) and O2 (~20%). In the cylinder of an automobile engine,...
-
Carlberg Company has two manufacturing departments, Assembly and Painting. The Assembly department started 11,000 units during November. The following production activity in both units and costs...
-
In a helium dilution refrigerator 3He are mixed in a special chamber to obtain extremely low temperatures. A Bainbridge mass spectrometer is used to measure the ratio of the two isotopes. (a) If the...
-
For each n 3 let P n be a regular polygon of n sides (for n = 3, P n is an equilateral triangle; for n = 4, a square). A Symmetry of P n is a bijection P n -P n that preserves distances and maps...
-
Could Figure P28.12 represent the magnetic field due to a bar magnet that has a rectangular cross section rather than a circular cross section? Data from Figure P28.12 N S
-
The Speedy Pizza Palace is revamping its order-processing and pizza-making procedures. In order to deliver fresh pizza fast, six elements must be completed. a. Construct a precedence diagram and...
-
6.Max Co is a large multinational company which expects to have a $10m cash deficit in one month's time. The deficit is expected to last no more than two months. Max Co wishes to resolve its...
-
Background: In the initial contract negotiation stage, the contract price with SM was $10.1 million in cash. However, as part of the final contract negotiations, SM agreed to give EYE SPY its old...
-
The reaction of sulfur dichloride and sodium fluoride yields sulfur tetrafluoride, disulfurdichloride, and sodium chloride. Write the balanced equation. What mass of sulfur tetrafluoride is formed by...
-
Many important chemical processes require two (or more) steps. One example is a process used to determine the amount copper in a sample. Many copper compounds, dissolved in water, will react with...
-
Find the one-sided Fourier sine transform of the function f(x) = e ax /x.
-
A diploid organism has a total of 14 chromosomes and about 20,000 genes per haploid genome. Approximately how many genes are in each linkage group?
-
A black female cat (XBXB) and an orange male cat (XOY) were mated to each other and produced a male cat that was calico. Which sex chromosomes did this male offspring inherit from its mother and...
-
On rare occasions, a human male is born who is somewhat feminized compared with other males. Microscopic examination of the cells of one such individual revealed that he has a single Barr body in...
-
What is the difference between an F+ and an Hfr strain? Which type of strain can transfer many bacterial genes to recipient cells?
-
Describe the characteristics of highly repetitive DNA sequences.
-
Ralph transfers property with an adjusted basis of $65,000 and a FMV of $70,000 to Lake Corporation in a Sec. 351 transaction. Ralph receives a stock worth $60,000 and a short-term note having...
-
SCHEDULE OF COST OF GOODS MANUFACTURED The following information is supplied for Sanchez Welding and Manufacturing Company. Prepare a schedule of cost of goods manufactured for the year ended...
-
For each of the following compounds determine whether it exhibits a cis configuration or a trans configuration or whether it is simply not stereo-isomeric. a. b. c. d. e. f. g. F
-
Identify the number of stereo-isomers that are possible for a compound with the following constitution: H 2 CCHCH 2 CH 2 CH 2 CHCH 2 .
-
Compound X and compound Y are constitutional isomers with molecular formula C 5 H 10 . Compound X possesses a carbon-carbon double bond in the trans configuration, while compound Y possesses a...
-
Galaxy Company has following balances from previous month-May: Cash: $900.000 debit balance, Bank: $800.000 debit balance, Goods: $400.000 debit balance A) General Journal Entries (85 pts) Galaxy...
-
A proton accelerates from rest in a uniform electric field of 600 N/C. At some later time, its speed is 1.42 x 106 m/s. (a) Find the magnitude of the acceleration of the proton. 5745367603 m/s (b)...
-
1. Rusties Company recently implemented an activity-based costing system. At the beginning of the year, management made the following estimates of cost and activity in the company's five activity...

Study smarter with the SolutionInn App


