More than 5 billion pounds of ethylene oxide, C 2 H 4 O, is produced annually. Ethylene
Question:
More than 5 billion pounds of ethylene oxide, C2H4O, is produced annually. Ethylene oxide is used in the production of ethylene glycol, HOCH2CH2OH, the main component of antifreeze, and acrylonitrile, CH2CHCN, used in the production of synthetic fibers and other chemicals. Ethylene oxide has an interesting cyclic structure.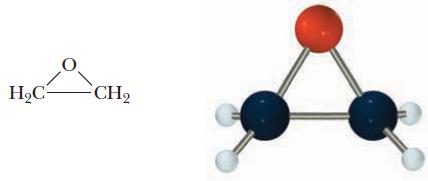
Draw the Lewis structures of ethylene oxide, ethylene glycol, and acrylonitrile, and give the hybrid orbitals on each central atom in these three molecules. Are any π bonds present in these molecules?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





