Nitrogen monoxide, NO, is a pollutant formed in running automobile engines. It reacts with oxygen in the
Question:
Nitrogen monoxide, NO, is a pollutant formed in running automobile engines. It reacts with oxygen in the air to produce nitrogen dioxide, NO2. Calculate the volume of NO2 gas produced and the volume of O2 gas consumed when 2.34 L NO gas reacts with excess O2. Assume that all volumes are measured at the same pressure and temperature.
Strategy
Volumes of gases combine in the same proportions as the coefficients in the equation.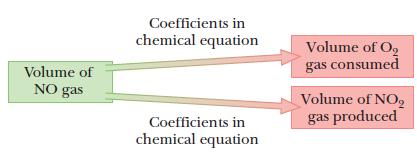
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





