One step in the production of nitric acid, a powerful acid used in the production of fertilizers
Question:
One step in the production of nitric acid, a powerful acid used in the production of fertilizers and explosives, is the combustion of ammonia.![]()
Use Equation 5.10, with the enthalpies of formation of these substances in Table 5.2, to find![]() of this reaction.
of this reaction.
Equation 5.10
![]()
Table 5.2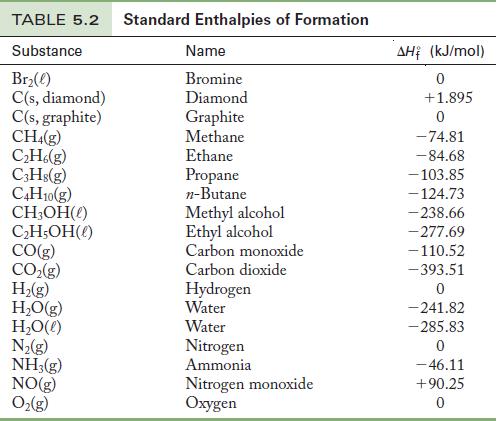
Strategy
Look up the standard enthalpy of formation of each substance in Table 5.2, recalling that the value of ![]() for any element in its standard state is zero. Multiply each value from Table 5.2 by the coefficients from the balanced chemical equation. Sum the resulting values for the products and subtract the resulting values of the reactants. When collecting terms together, watch the signs of each
for any element in its standard state is zero. Multiply each value from Table 5.2 by the coefficients from the balanced chemical equation. Sum the resulting values for the products and subtract the resulting values of the reactants. When collecting terms together, watch the signs of each ![]() value.
value.
Step by Step Answer:

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball





